Breaking: Eligo Bioscience Reports First-Ever in Vivo Microbiome Base Editing

Research to understand the importance of the human microbiome in health and disease has revealed several ways in which commensal bacteria can impact our health.
For instance, gut bacteria have been shown to influence the outcome of certain cancer immunotherapies, bacterial peptides produced in the microbiome can drive acute and chronic disease including neurodegenerative diseases, autoimmunity and cancer, and certain gut bacteria can alter drug effectiveness through modification or sequestration.
The human microbiome is a hot gene-editing target
The examples above highlight the therapeutic potential of selectively targeting certain bacteria within the microbiome, and several attempts using phage or bacterial conjugation to deliver CRISPR- or base-editing cargo are reported in the literature.
However, while previous studies show that microbiome gene editing is possible, their efficiency and therapeutic relevance have been hampered by poor delivery, limited targeting range, or low editing efficiency within the target bacterial population (1, and references therein).
Now, those challenges have been solved by the team at Eligo Bioscience, who have developed an engineered phage-derived particle capable of delivering a base editor to a single E. coli strain in the mouse gut. Remarkably, they report almost 100 % editing efficiency in the target strain among hundreds of bacterial species present in the mouse gut. Using their highly efficient strategy, the team could disrupt antibiotic-resistance genes or virulence factors with extreme precision by creating single-base pair edits in the encoding genes.
“It's really the first time we can envision creating therapies that can precisely inactivate the bacterial gene(s) driving disease while having close to no impact on the microbiome composition and function. This could be a paradigm shift, switching from microbiome therapies to gene editing therapies targeting bacterial genes - this strategy broadens the landscape of addressable therapeutic targets in the gene editing field”Xavier Duportet, CEO Eligo Bioscience
In situ targeted base editing of bacteria in the mouse gut
To achieve efficient editing and robust delivery, the team engineered a phage-derived capsid that efficiently expresses a base editor but which is not replicated in recipient bacteria. The vector design strategy, which is detailed in their manuscript (1), allowed them to introduce stable edits to >90 % a target E. coli population colonising the mouse gut without the need for selection pressure or maintenance of a transgene. The vector was administered orally to mice as a potential single-dose microbiome therapy.
**CRISPRMED25 - The 2nd CRISPR Medicine Conference, Copenhagen, Denmark, April 7-11, 2025**
Learn about the latest discoveries in base editing at the CRISPRMED25 Conference in Copenhagen, Denmark, April 7-11, 2025.
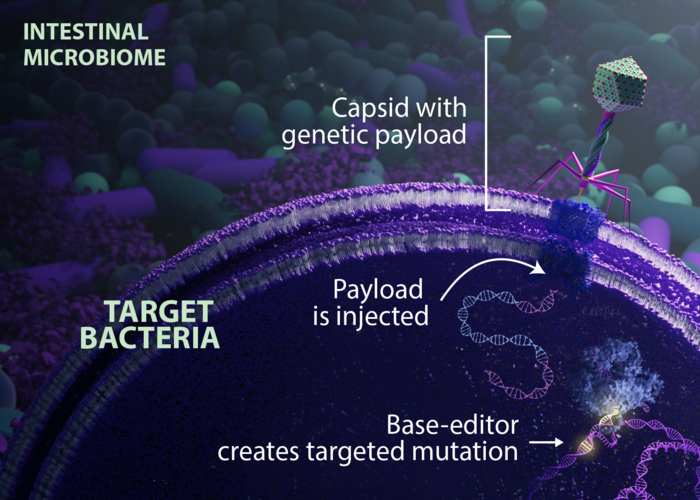
In summary, the team observed that base editing of a β-lactamase gene (which confers multidrug resistance to the beta-lactam antibiotics) in the target E. coli strain resulted in a median editing efficiency of 93% of the target population, and that edited bacteria remained stable in the mouse gut for at least 42 days following a single dose of treatment. When they applied this strategy to edit several genes of therapeutic relevance in E. coli and Klebsiella pneumoniae strains in vitro, they could demonstrate in situ editing of a gene involved in the production of curli (a biofilm-promoting protein) in a pathogenic E. coli strain.
A new era of microbiome therapies?
The findings shared today open new doors within microbiome editing for research as well as therapeutic development. According to its press release, Eligo Bioscience is already applying the technology to develop new therapies for various undisclosed conditions that affect millions of people globally.
Speaking with CRISPR Medicine News about the impact of the findings, Eligo Bioscience' CEO Xavier Duportet said:
»It's really the first time we can envision creating therapies that can precisely inactivate the bacterial gene(s) driving disease while having close to no impact on microbiome composition and function. This could be a paradigm shift, switching from microbiome therapies to gene-editing therapies targeting bacterial genes - this strategy broadens the landscape of addressable therapeutic targets in the gene-editing field«.
References:
- Andreas K. Brödel, Loïc H. Charpenay, Matthieu Galtier et al. (2024). In situ targeted base editing of bacteria in the mouse gut. Nature.
Tags
ArticleBreaking newsNewsMicrobiomeBase editorsEligo Bioscience
CLINICAL TRIALS
Sponsors:
Children's Hospital of Fudan University
Sponsors:
CorrectSequence Therapeutics Co., Ltd







