Clinical Trial Update - Meganuclease-Edited CAR T-Cell Therapies
The latest trial is sponsored by Precision BioSciences (US) and it will evaluate the safety and efficacy of PBCAR19B, a gene-edited allogeneic (donor-derived) anti-CD19 CAR T cell therapy for relapsed or refractory NHL.
PBCAR19B is a so-called 'stealth cell' anti-CD19 CAR T product that is designed to prevent immune rejection by recipients. The new therapy is engineered using Precision BioSciences’ next-generation meganuclease platform called “ARCUS”. The ARCUS platform produces meganucleases (See Fact Box) with customised activity and specificity, which are capable of distinguishing target sites that differ by only 1 base pair.
FACT BOX: Meganucleases
Meganucleases are a group of naturally occurring and highly specific restriction enzymes that were first discovered and recognised to have gene-editing potential in the 1990s. However, in spite of their high specificity and low cell toxicity, their natural diversity is limited making it difficult to realise their gene-editing potential for broad therapeutic purposes.
In recent years, companies have developed platforms to engineer tailor-made meganucleases with specificity for desired sequences through modifications to the enzyme’s recognition site or by mixing and matching domains from distinct meganucleases into recombinant versions with the sought after properties.
PBCAR19B – For Improved Persistence of Allogeneic CAR T Cells
PBCAR19B is designed to improve allogeneic CAR T cell persistence by preventing rejection by recipient T cells and natural killer (NK) cells. PBCAR19B cells are developed through three meganuclease-mediated modifications.
Firstly, an anti-CD19 CAR construct is inserted into the native T cell receptor (TCR) locus. Disruption of the donor's native TCR mitigates the risk of graft-vs-host disease that may occur if histo-incompatibility between donor and recipient causes the infused cells to reject their new host.
The PBCAR19B vector also harbours a short hairpin RNA that suppresses beta-2 microglobulin (B2M) expression. B2M is a component of the Class 1 major histocompatibility complex (MHC) that is present on the surfaces of all T cells. B2M disruption eliminates the expression of all MHC I molecules on CAR T cells, thus reducing the risk of self-presenting to recipient T cells, allowing persistence in their new host. This approach is being investigated in a number of CAR-T cell programs and is reviewed elsewhere.
Lastly, PBCAR19B cells are engineered to express the conserved HLA-E transgene. While B2M disruption eliminates all MHC I molecules to boost persistence, it also leaves the CAR T cells vulnerable to NK cells. HLA-E surface expression in the absence of all other MHC molecules prevents NK-mediated lysis without stimulating allogeneic T cells. This strategy is being explored for the creation of universal donor cells for CAR T cancer therapy.
PBCAR19B Trial for Non-Hodgkin Lymphoma
In pre-clinical studies, PBCAR19B was found to delay both T cell and natural killer cell-mediated allogeneic rejection. Precision BioSciences reported in January 2021 that it had been granted IND clearance for PBCAR19B from the U.S. Food and Drug Administration (FDA).
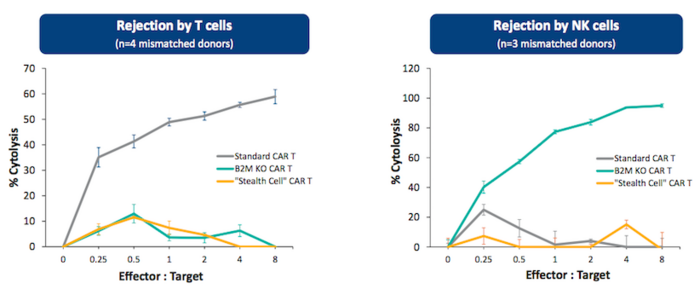
The trial is a Phase 1 non-randomised open-label dose-escalation and dose-expansion study that is expected to begin in May 2021 with 24 adult participants who have previously undergone two standard treatment regiments for NHL. Participants will received a single dose of PBCAR19B. Primary outcome measures include finding the maximum tolerated dose and assessment of adverse events related to dosing.
In addition to the trial discussed here, Precision BioSciences is sponsoring 3 other trials for meganucleuase-engineered CAR-T cells for multiple myeloma (MM), B-cell Acute lymphoblastic leukaemia (ALL) and Non-Hodgkin Lymphoma (NHL) or Chronic Lymphocytic Leukaemia (CLL) or Small Lymphocytic Lymphoma (SLL). You can read more about these trials in our previous post.
For a complete overview of current gene editing clinical trials, check out CRISPR Medicine News' Clinical Trials Database.
Tags
ArticleNewsin vivoNon-Hodgkin Lymphoma, NHLCAR-TMeganucleasesInstitut De Recherches Internationales Servier Iris, SARL.Precision BioSciences, Inc.TrialsClinicalIND - Investigational New Drug
CLINICAL TRIALS
Sponsors:
Suzhou Maximum Bio-tech Co., Ltd.
Sponsors:
Zhejiang University







