CMN Weekly (18 August 2023) - Your Weekly CRISPR Medicine News
By: Gorm Palmgren - Aug. 18, 2023
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...
Top picks
- Inspired by viral invasion, American researchers have engineered a DNA framework that can control CRISPR-Cas9. The DNA framework state machine (DFSM) can navigate living cells with temporal Precision, and by responding to specific molecular cues, the DFSM can assume multiple reversible structural states. Notably, this enables controlled CRISPR-Cas9 targeting of chromatin loci, illuminating prospects for intelligent theranostics in complex biological environments.
- American scientists have utilized CRISPR-Cas9 to treat dominant hearing loss mutations. Using a mouse model, they employed liposome-mediated delivery to edit the responsible gene, Atp2b2. In double-mutation cases, this leads to outer hair cell survival, functional recovery, and even partial restoration. This breakthrough paves the way for gene editing as a potential treatment for hearing loss.
Research
- A landmark study identifies DNA-dependent protein kinase (DNA-PK) as a critical target for optimizing CRISPR-Cas9 genome editing. AZD7648, a DNA-PK inhibitor, and Polϴ inhibition synergistically improve templated insertions, achieving 80% efficiency with minimal off-target effects. This advancement significantly enhances gene editing accuracy and the potential for genetic disease treatments.
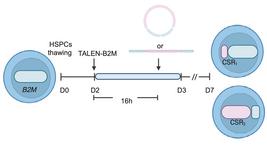
- Researchers at Queen Mary University of London have demonstrated that epigenomic editing is heritable during human haematopoiesis. They used dCas9-effector fusion for locus-specific epigenetic editing in haematopoietic stem and progenitor cells (HSPCs). Results showed that the epigenomic changes persisted during myeloid differentiation, reducing expression of the p15 target gene.
- Excision BioTherapeutics' experimental CRISPR therapy EBT-101, now in clinical phase 1 trial for treating HIV in humans, has shown positive results for targeting SIV (simian immunodeficiency virus) DNA in rhesus macaques. Following a single IV dose of EBT-001, broad and effective distribution was achieved without off-target effects, and the animals showed improved health markers, especially at higher doses.
- Researchers in the USA have demonstrated a safe and effective brain gene editing technique by using focused ultrasound (FUS) to transiently open the blood–brain barrier (BBB) to transport intravenously AAV-delivered CRISPR-Cas9 machinery to the brain. In an in vivo mouse model, they achieved 25% gene editing efficiency of neurons.
- Researchers in Singapore describe a new approach to CRISPR-Cas9 gene editing that harnesses a phase-separating peptide with redox-triggered release properties. The peptide efficiently delivers all CRISPR components - plasmid DNA, mRNA/sgRNA, and the ribonucleoprotein complex - and surpasses existing techniques, exhibiting superior transfection and editing efficiency.
- A promising advancement in treating rare pediatric blindness emerges as American scientists have delivered base editors to mice's retinal pigmented epithelium (RPE) using silica nanocapsules. These capsules precisely correct a genetic mutation responsible for Leber Congenital Amaurosis (LCA16). The treatment restores functional vision in the mouse model, bolstering the potential of nonviral genome editing for inherited disorders.
Industry
- Precision BioSciences has completed a strategic transaction with Imugene for global rights to azer-cel, Precision's lead allogeneic CAR T candidate for cancer treatment. According to the deal, Precision can receive up to $227 million in upfront economics and milestone payments in addition to double-digit royalties on sales.
- Verve Therapeutics reports second quarter 2023 financial results with a cash position of $463 million and a net loss of $54 million. The company also reports progress for its three experimental cholesterol-lowering base-editing treatments: VERVE-101, VERVE-102, and VERVE-201.
Reviews
- Heritable genome editing: ethical aspects of a developing domain. This mini-review summarizes the morally relevant aspects of the rapidly developing discipline of human germline genome editing (GGE), focusing on reproductive applications and with particular attention to the ethical questions about how this technology may affect the interests of those who might become the result of genome editing.
- Breakthrough in CRISPR/Cas system: Current and future directions and challenges. This review highlights the progress made in CRISPR technology, including multiplex editing, base editing (BE), and prime editing (PE), as well as the challenges and potential delivery mechanisms.
Conferences and meetings
- The conference "Genome Engineering: CRISPR Frontiers" at the Cold Spring Harbor Laboratory is ongoing. The list of abstracts has been published and is readily available for everyone.
Huh, heh, wow
- A News story in Nature Biotechnology unfolds how a new generation of companies is using CRISPR to upgrade the production of insect larvae as a protein source for animal feed, fertilizer, biofuels and even as ingredients for burgers and shakes. CRISPR gene editing is used for yielding strains with higher lipid content, custom proteins, larger larvae, a more extended larval period and increased resilience under stress.
- A News & Views article in Nature Microbiology discusses CRISPR-influenced symbiosis in archaea. CRISPR systems canonically confer microorganisms protection against invading viral DNA, plasmids and mobile genetic elements. However, a new multi-omics investigation of deep subsurface archaeal communities suggests that archaeal CRISPR systems might target other archaeal parasites or force a transition from parasitism to mutualism.
News from CRISPR Medicine News
- On Monday, we reported how CRISPR-edited dual stem cell therapy offers fresh hopes for brain metastases. In an interview with our reporter, Khalid Shah at Harvard explained his team's new innovative CRISPR therapy to treat deadly metastatic brain melanomas.
To get more of the CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
CLINICAL TRIALS
IND Enabling
Phase I
Phase II
Phase III
Gastric Cancer and Colorectal Cancer, CRC, (NCT07166263)
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
IND Enabling
Phase I
Phase II
Phase III
Relapsed or Refractory Acute Myeloid Leukemia, AML, (NCT06541444)
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
IND Enabling
Phase I
Phase II
Phase III