CRISPR and Universal CAR-T cells
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...
In the past episode, we took a leap of faith and talked about cancer immunotherapy, particularly CAR-T cells.
Since there is still much to talk about, we encourage you to check out that episode before you keep reading!
Now let’s go back to CRISPR/Cas and different ways we may leverage it to make CAR-T cell technology better and more effective.
When it comes to CAR-T technology we can identify two main challenges: i) time required to generate CAR-T cells and ii) CAR performance in hard-to-kill tumors.
Both are equally important and in this article, we will tell you about the first one! For the second one, you’ll have to wait for next week.
Tick-tock: the run against time
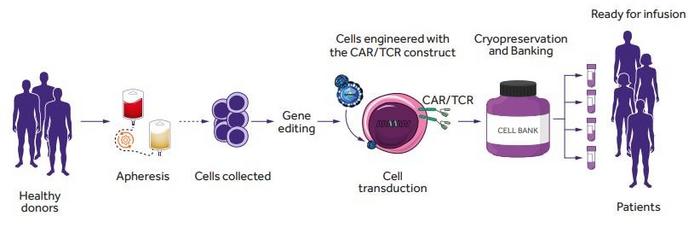
The average time to generate a [1].CAR T cell product from the patient’s own T cells may span from 9 to 14 days or even longer [1]. So here you are sitting in a hospital room, lying down as the doctors take blood from you and they rush it to the lab. Here they will isolate your T cells and kickstart the process of generating your tailor-made CAR-T cells, called autologous CAR-T cells because, as we said, they come from your own body.
The clock is ticking, and you hope you can last until the CAR-T cells are ready and that nothing goes sideways with the manufacturing process, which happens in 2-10% of cases[1].
Sometimes, tough, time is not on your side. And sometimes the cancer has deteriorated your T cells – like in T cell leukemia – so much that you don’t have enough to make a CAR out of them! So, wouldn’t it be awesome to mass produce CAR-T cells that can be use for everyone, so we can have a bag of fresh CAR-T cells taking a nap in the fridge ready to be deployed as soon as there is a need for them? This is the question many scientists asked themselves, and technologies like CRISPR/Cas9 may provide a solution.
Mix and match: avoiding rejection is the key to stock CAR-T therapies
How do we do this? Well, the problem here is very similar to what happens with organ donation and transplantation: we need a good match.
You see, your immune system is your best friend, but sometimes it can get in your way. It does not mean you ill, but sometimes it is just a bit too overprotective.
In this case, if CAR-T cells coming from another person are seen as “foreign” by your immune system, they will be rejected. And our immune system is as polite as a Husky with its pups, and will attack the CAR-T cells treating them like they are an infectious agent. So much for the help! In medical terms, this is not called Husky Rage but “Host-vs-Graft Disease” (GvHD) and it is exactly the same thing that happens when a transplanted organ is rejected.
On the other hand, CAR-T cells – at the end of the day – have exactly the same right to reject you. Yep, welcome to the modern age where everything is mutual! And yes, rejection is part of it. In a similar way to GvHD, CAR-T cells that find themselves interacting with another person’s cells can consider them as the intruder. I think you know what happens next, things start getting nasty. This is called “Graft-vs-Host Disease” and it can be very serious, particularly if you are not doing that well already because of the cancer taking a toll on you.
Well, you know already where we are going with that.
Editing immune compatibility to save lives
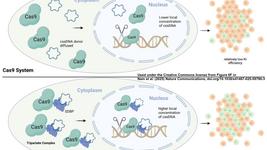
As far as GvHD is concerned, the key is to take out the ability of CAR-T cells to recognize “foreign” antigens. Luckily, they don’t need this function to do their job. So we can knock out the TRAC locus, which codifies for the alpha-chain, one of the two main components of the T cell receptor (TCR). Without the TCR, T cells cannot bind to the antigen offered by antigen-presenting-cells (APCs). Therefore, they can only recognize the target we give them via the CAR! So, TRAC KO CAR-T cells (yes, this is getting funny, we know) cause negligible or no GvHD at all!
But how about the HvGD? This matter is a bit more complicated and still quite debated. You see, cells express on their surface molecules which identify them as part of your organisms. They are like a finger print for the immune system, if it is wrong….boy oh boy you better run! This fingerprint expressed on all nucleated cells is part of the Major Histocompatibility Complex I (MHC I).
In this case, we will also use a CRISPR knockout strategy, but the target we will go for is B2M. This subunit stabilizes the MHCI molecules exposed on the cell surface. So, if we take out the scaffold, the rest will fall with it. This way, we don’t need to think about “person specific targets”.
Is this enough? Well, where there is one, there are two. And in this case, T cells (or CAR-T cells) when activated – like it happens when they encounter a cancer cells – can upregulate a set of molecules which are part of the -you guessed it- Major Histocompatibility Complex II (MHC II). However, as their expression is subjected to the CAR-T activation status –and other parameters – it is not as critical as MHC I. So, its abrogation by CRISPR is not standard when it comes to Allogenic CAR T development.
Even more debated is the development of strategies to protect the CAR-T from Natural Killer (NK) cells. These immune cells are attracted by cells which do not display MHC I and/or MHC II molecules anymore, and they are a bit extra with their reaction. “You know, healthy cells should have them, so why don’t these ones have any? Let’s kill them first and ask later”. Once again, our immune system is very overprotective! To circumvent this, some research teams are also expressing together with the CAR genes that mask them from the NK cells. However, this remains a strategy that is not used that often [2].
So here you have it, by knocking-out the TCR expression and interfering at least with MHC Class I, we can generate what we usually refer to as “off-the-shelf” CAR-T cells. These CAR-T cells are ready to use and can be infused to the patient immediately compared to autologous CAR-T cells. A number of companies – like CRISPR Therapeutics – are working exactly on this! Also, if you remember Allogenic T cells – engineered via Base-Editing – were administered to a girl suffering from T cell leukemia, saving her life!
In the following episode we will have a look at how CRISPR can help the CAR T cells when they are facing an even stronger opponent!
To get more CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.