CRISPR clinical trials overview 2020
It's an exciting time for CRISPR gene editing therapy. 2019 saw results from the first human clinical studies and 2020 have already seen the CRISPR gene editing tools inserted directly into the human body for the first time. Many more clinical studies are in the pipeline and to stay on top of the rising wave we have made a comprehensive overview.
We have combed the US and EU clinical trials database for CRISPR gene editing trials. We are also adding other gene-editing modalities that can do much of the same as CRISPR such TALENs and zinc finger nucleases (ZFN). As new studies enter clinical trials, and as the current trials progress, we will update our overview.
Here are the main trends
First, we find most of the clinical trials are registered in the United States and China, with just a handful of trials so far in Europe.
China first
China has the lead with ten registered CRISPR trials and began in 2016 with cancer immunotherapy treatments. The studies enroll 10-80 patients and potentially more than 250 Chinese cancer patients have already received CRISPR gene-editing treatments. Unfortunately, very little data has been published from China, and also concern has been raised on the safety and rigour of the trials. The scandal 'CRISPR babies' trial by He Jiankui has not helped either. So it's difficult to know what the trials genuinely are and how much trust to put in them.
Low risk and easy delivery
Across all gene-editing trials at this stage, researchers are aiming for the low hanging fruits of treatments that are 'easy' to treat. That is low risk and easy delivery approaches that follow in the steps of other treatments such as immunotherapy. So most are not entirely new treatments but allows researchers to assess CRISPR as a gene-editing tool in humans in a careful way.
Most are ex vivo...
Except for a few, the gene-editing studies are all 'ex vivo', meaning the editing can be done on cells in a very controlled way in the lab outside the human body, without the risk of dangerous immune reactions to viral vectors or the bacterially derived CRISPR components.
The gene-editing of disease most people think about, are the 'in vivo' treatments, where the gene editing tools are injected directly into the body and cure the disease by correcting mutations in a specific tissue or cells. These studies are so far the exception.
...but a few are in vivo
There is a few in vivo studies targeting the eye, the skin and the liver. Some are in China, where the gene-editing tools are delivered in a gel to treat HPV induced cervical cancer.
The other is a trial by Sangamo Therapeutic for treating haemophilia by intravenously delivered zinc finger nucleases (ZFN) to induce insertion of a corrective copy of the blood clotting Factor IX into liver cells.
The most recent in vivo trial is a CRISPR treatment for an inherited form of blindness run by Editas Medicine in collaboration with Allergan. Just a few weeks ago, they announced the exciting news that the first patient had received the treatment by injection directly into the eyes to correct mutations in the photoreceptor gene CEP290.
The study will be followed closely and will be a milestone for the field if the treatment works as hoped.
Much more to come
Where the clinical studies so far are focusing on diseases that are relatively 'easy' to treat - easily accessible for delivery and low risk for immune reactions - the future will undoubtedly see more in vivo treatments.
The technologies are developing fast for example to allow more precise gene editing with little risk of off-target mutations, and better delivery vehicles that can target specific tissue and cell types, and not risk causing immune reactions.
This year and the coming years are sure to be full of exciting new developments, and so far the CRISPR technology looks to be living up to the hype. Keep in mind that all the clinical studies are in the early stages, and even if they are successful, it will still be several years before they get approval.
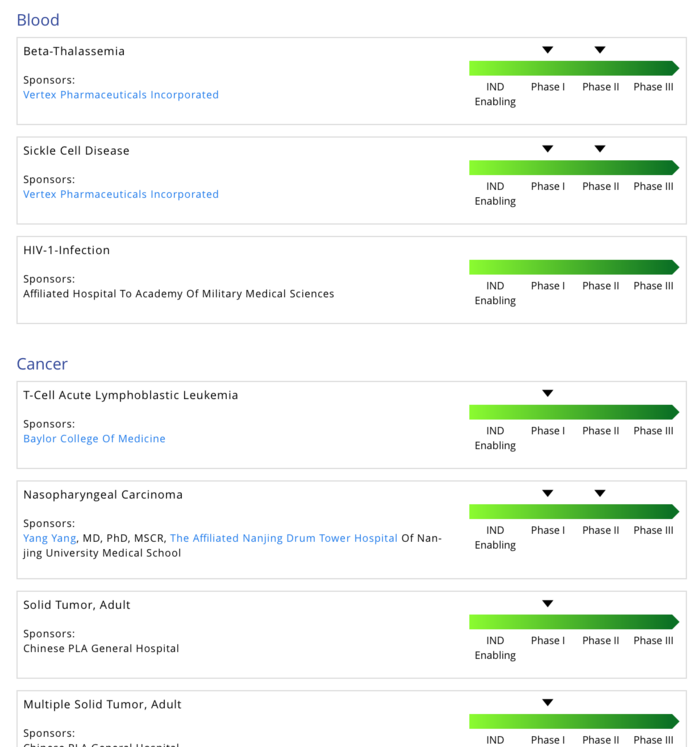
Below follows a description of the trials, which fall into four main areas: Antibacterials, blood, cancer, and eye.
Cancer clinical studies
Currently, gene-editing clinical studies for cancer treatments are variants of what is known as CAR (Chimeric antigen receptor) T-cell therapy and PD-1 (Programmed cell death protein 1) immunotherapy.
They are based on ex vivo gene editing where the immune system T-cells are edited in the lab, expanded and put into patients to hunt down and kill cancer cells.
In CAR-T therapy, the T-cells are transduced with a genetically engineered T-cell receptor that recognizes specific cancer cells.
The treatment strategy is to remove the PD-1 protein, which is 'safety switch' on the T-cells, that the cancer cells can turn on to protect themselves. PD-1 is the same protein that is blocked by antibodies in immunotherapy, so the gene-editing strategy builds on the same idea. On top of that, researchers may also remove the endogenous T-cells receptors which might interfere with the engineered CAR-T receptor.
Finally, the twice engineered T-cells are expanded and put into the cancer patient.
Late last year, a milestone was reached with the first clinical trial data from three late-stage cancer patients. Reassuringly the treatment seemed to be safe, showing that multiple highly specific genome editing can be delivered in humans without obvious side-effects. However, it is still too soon to say whether the treatment will be effective against advanced cancer.
A new variant of the strategy is to generate 'universal' T-cells from healthy donors rather than isolate T-cells from cancer patients, who might have very few T-cells. To make universal researchers need to prevent the T-cells from attacking the host (graft vs host) by gene editing.
The number of CAR-T clinical trials have exploded in recent years to over 500 globally and the gene-editing enhanced CAR-T, could very well follow that trend in the coming years, if the genetic PD-1 blocking works as well as current immunotherapies treatment and the trials are shown to be safe.
Blood clinical studies
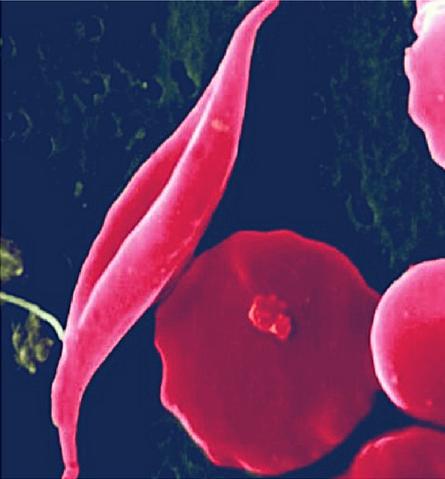
Blood clinical trials are similar to the cancer trial in that blood cells can be easily removed and edited ex vivo. There are trials for four different blood disorders: sickle cell disease, beta thalassemia, HIV and haemophilia.
Sickle cell disease and beta-thalassemia are both disorders that affect haemoglobin, the molecule in red blood cells that delivers oxygen to cells throughout the body. In sickle cell disease mutations cause atypical haemoglobin molecules, which can distort red blood cells into a sickle, or crescent, shape. The misshapen blood cells can block blood vessels and slow blood flow causing pain, anaemia, strokes, and organ damage.
In beta thalassemia mutations reduce the production of haemoglobin causing anaemia and fatigue. Both disorders can be fatal.
Two treatments are currently in clinical studies by CRISPR Therapeutics and its partner Vertex Pharmaceuticals. They infuse a CRISPR therapy called CTX001 in patients in the US, Canada, and Europe.
The treatment strategy does not aim to fix the faulty gene per se but instead aim to reactivate fetal haemoglobin in blood stem cells, that normally is only expressed when the fetus is in the womb.
The first encouraging preliminary results from two patients were announced in November 2019, showing the treatment so far to alleviate symptoms and have few side effects. It will be exciting to follow these studies with new results may be announced in 2020 and 2021.
Other trials of blood disorders are treatments for HIV - the human immunodeficiency virus. HIV harms the immune system by destroying the white blood cells that fight infection and puts patients at risk for severe infections and certain cancers.
There are currently one CRISPR-based trial in China and several ZFN-based trials in the US. The treatment strategy is to remove a protein called CCR5, which HIV uses to enter the cells. Studies have shown people born without CCR5 on their cells remain healthy and are resistant to infection with HIV.
To do this, blood stem cells are isolated from the patients, edited ex vivo, and returned to the subjects.
There is also an 'in vivo' clinical study. A treatment by Sangamo Therapeutics for the bleeding disorder haemophilia B. The treatment called SB-FIX aims to deliver a corrective copy of the Factor IX transgene using ZFN's and AAV vectors to target liver cells. If the treatment works, it will provide a permanent, liver-specific expression of the Factor IX blood clotting factor.
More trials are expected in blood disorders, and it will be interesting to follow how efficient the treatments are, how well the edited cells survive in the bone marrow or liver, and if they produce enough protein to manage the disease.
Eye clinical trials
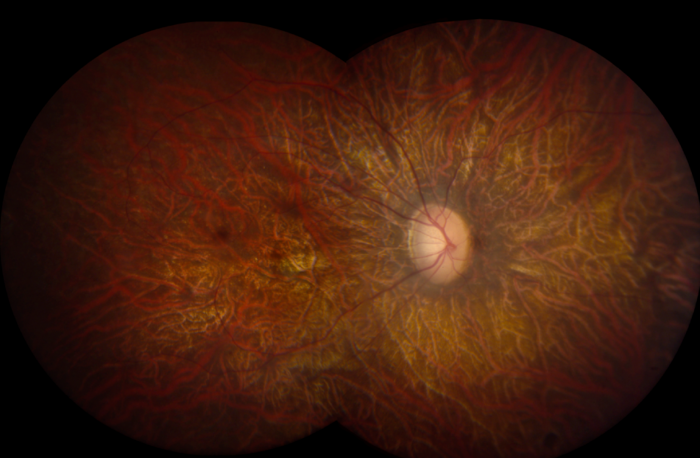
There is just one clinical study on eye disorders so far, but a potentially a very influential one.
The trial is a CRISPR gene-editing treatment for the most common cause of inherited childhood blindness: Leber Congenital Amaurosis (LCA). It is an eye disorder that primarily affects the retina, which is the specialized tissue at the back of the eye that detects light and colour.
LCA is caused by mutations in the photoreceptor gene CEP290, which causes degeneration in photoreceptor cells responsible for experiencing vision.
The treatment aims to correct the mutation and thereby restore normal protein expression, photoreceptor function, and ultimately, vision. The CRISPR components are packed into non-pathogenic AAV viral vectors that are injected directly into the patient’s eye.
In March Editas Medicine, who is running the trial in collaboration with Allergan, announced the exciting news that the first patient had received the treatment.
What is particularly noteworthy is that this is the first time the CRISPR-Cas9 technology is deployed directly into the human body. If successful, the trial will represent a major milestone for the gene-editing field by being the first demonstration of curing genetic disease by editing a faulty gene in the body.
Antibacterial studies
There is just one clinical trial, and it is an outlier in that it does not treat human disease by editing human genes, but by editing bacterial genomes. However, it is a new type 'antibiotic', and more antibacterial clinical trials are on the horizon to fight infections, which is good news in a time where multi-drug resistance is spreading.
The first antibacterial CRISPR clinical study is a treatment for a common infection: Urinary tract infections. It is a CRISPR enhanced phage therapy run by Locus Biosciences, which apply a cocktail of bacterial viruses (phages) that infect and kill the target E. coli bacteria. But to tip the scales, the phages also delivers CRISPR-Cas3, which is a 'DNA-shredder' that destroys the E. coli genome.
If the treatment works, it can be the first of many in a new antibacterial paradigm, where the bacteria causing the infection are killed specifically rather than in broad sweep with traditional antibiotics.
Tags
CLINICAL TRIALS
Sponsors:
Suzhou Maximum Bio-tech Co., Ltd.
Sponsors:
Zhejiang University







