CRISPR Diagnostics Update: Cas12a-based SARS-CoV-2 Detection
This week’s update describes Specific Enhancer for Detection of PCR-amplified Nucleic Acids (SENA), and the clinical study that was performed to assess its utility as a confirmatory diagnostic tool for COVID-19 in China.
In brief, SENA combines the transcleavage acitivty of Cas12a with the sensitivity offered by real-time PCR to verify SARS-CoV-2 RNA in infected individuals.
SENA was developed by scientists from various research institutions and hospitals in China, and Weiren Huang, who is affiliated with Shenzhen Second People’s Hospital and other institutes, led the study which is described in a publication in EBioMedicine.
SENA exploits the transcleavage activity of Cas12a
SENA builds upon a strategy that is related to that seen in other CRISPR-Cas12a-based diagnostics, e.g., DETECTR (Mammoth Biosciences) and HOLMES, developed by Chinese researchers in 2018.
Cas12-based detection generally exploits the inherent ability of Cas12a to non-specifically cleave single-stranded DNA (ssDNA) reporter molecules upon specific recognition and cleavage of RNA-guided target DNA sequences. This non-specific cleavage activity is known as transcleavage.
By using a fluorophore quencher (FQ)-labelled ssDNA reporter molecule that emits a fluorescent signal upon cleavage, which only happens when the target is present in the sample, one can sensitively and specifically detect the target pathogen.
The real-time PCR 'grey zone' problem
In most of the world, real-time PCR detection is used as the principle tool for SARS-CoV-2 diagnosis, and this method is recommended by the American Centers for Disease Control and Prevention (CDC), the World Health Organization (WHO), and within the EU.
The main readout for real-time PCR is a cycle threshold or Ct-value. The Ct is the PCR cycle number at which the fluorescent signal emitted by a given PCR reaction reaches the threshold line.
The so-called real-time PCR ‘grey zone’ is a challenge in real-time PCR-based pathogen diagnosis in general, and this occurs when a test sample yields a high Ct-value, which is close to the cut-off for what can be considered positive. Standard real-time PCR kits for SARS-CoV-2 detection include 2 target amplicons to try and mitigate the grey zone issue, but sample re-collection and retesting are required in cases where real-time PCR results are inconclusive.
SENA was therefore developed as a simple and relatively cheap method to solve the “grey zone” issue by correcting false positive or false negative diagnoses.
How does SENA work?
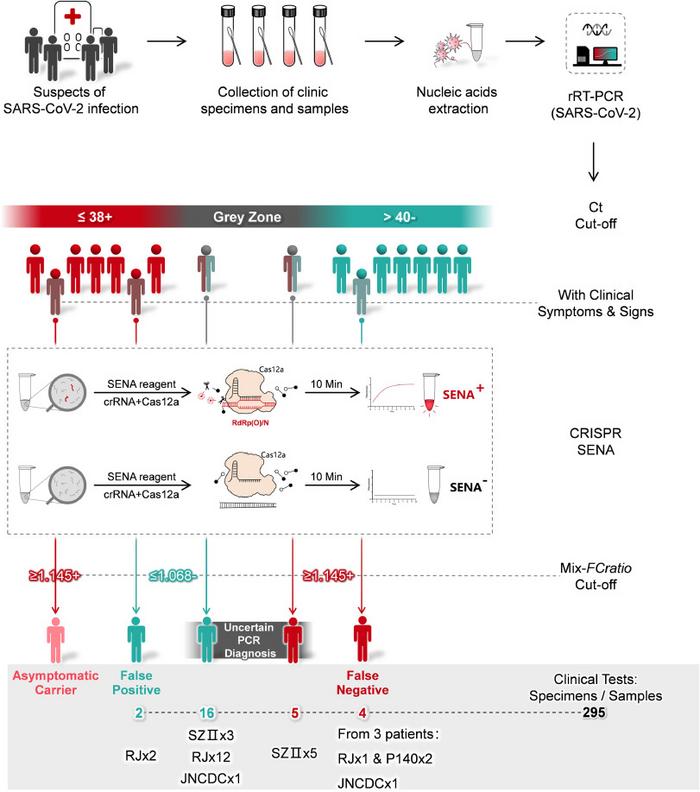
In the SENA workflow, a standard real-time PCR reaction is first performed on test samples using fluorescently-labelled oligonucleotide probes and primers that bind fragments of the Orf1ab (O) and N regions within the SARS-CoV-2 genome.
Following real-time PCR amplification, samples deemed positive, negative or in the grey zone, according to the resulting Ct-values, are mixed with the SENA reagent, which consists of Cas12a and a gRNA.
If the target amplicon (from SARS-CoV-2) is present in the mixture, Cas12a will bind it with the help of the gRNA, and then cleave it to release a fluorescent signal. The scientists behind SENA have dubbed this signal as the ‘SENA readout’. The gRNAs used in the SENA reagent were carefully optimised so as not to cleave the PCR probes or primers and yield a false-positive result.
SENA performed well in clinical study
The researchers compared the limit of detection (LoD) of SENA with that of three real-time PCR COVID-19 test kits available in China. While the LoD of the SENA procedure varied depending on the real-time PCR kit it was paired with, it was generally two copies less than the corresponding real-time PCR kit.
Using 295 clincal specimens throughout several hospitals and centres for disease control in China, the researchers identifed 4 false-negative and 2 false-positive results that resulted from testing with three real-time PCR kits. The clincal specimens included pharyngeal and nasopharyngeal swabs from individuals suspected to be infected with SARS-CoV-2. In addition, SENA resolved 21 specimens that were in the grey zone following real-time PCR determination. During the clinical study, the researchers also made observations that suggest that SENA, because of its high sensitivity, may also be useful in monitoring recovering COVID-19 patients for viral clearance.
The researchers note that despite its utility, SENA also has a grey zone and that clinical symptoms and next-generation sequencing-based methods may be useful in resolving samples that fall within this zone. The authors also note that to avoid aerosol contamination, SENA should be conducted in a clean environmnt that is phsyically separated from the real-time PCR testing area.
Related content
If you want to get an overview of some of the other ongoing CRISPR-based COVID-19 detection strategies, you may like our previous COVID-19 diagnostics roundup.
For a complete overview of current gene editing clinical therapeutic trials as well as diagnostic trials, check out CRISPR Medicine News' Clinical Trials Database.
Tags
CLINICAL TRIALS
Sponsors:
Wave Life Sciences Ltd.