CRISPRMED24 is a wrap, and here's to CRISPRMED25!
And just like that, CRISPRMED24 is a wrap! What an amazing week it has been for us and for the global CRISPR Medicine community!
What started out as a crazy idea last summer has turned out to be the most exciting and rewarding project CRISPR Medicine News has ever undertaken and we are already planning CRISPRMED25!
CRISPR MED24 Virtual
CRISPRMED24 kicked off virtually on Monday 22nd, where more than 100 delegates joined in from more than 35 countries to hear about how epigenetic editing is being used to develop a new treatment for chronic hepatitis B virus infection, a novel PCR-free assay to measure the outcome of gene-editing experiments, new gene-editing approaches to tackling cancer and genetic diseases, and how the European Medicines Agency is supporting the development of medicinal products using genome editing.
Our interactive poster session also worked really well and we are delighted that the virtual event created the possibility for so many people to take part in CRISPRMED24.
Exhibitors
Tuesday morning bright and early saw the arrival of our sponsors from all over the world to our conference venue Øksnehallen, a former market hall located on Halmtorvet in the Vesterbro district of Copenhagen.
The atmosphere was great as everyone got busy setting up their exhibiton booths to showcase the latest products and services within gene editing tools and analysis, DNA and vector synthesis, functional genomics, cell and -omics analysis, and much much more.
Many thanks to each and every one of you, and hope to see you again next year!

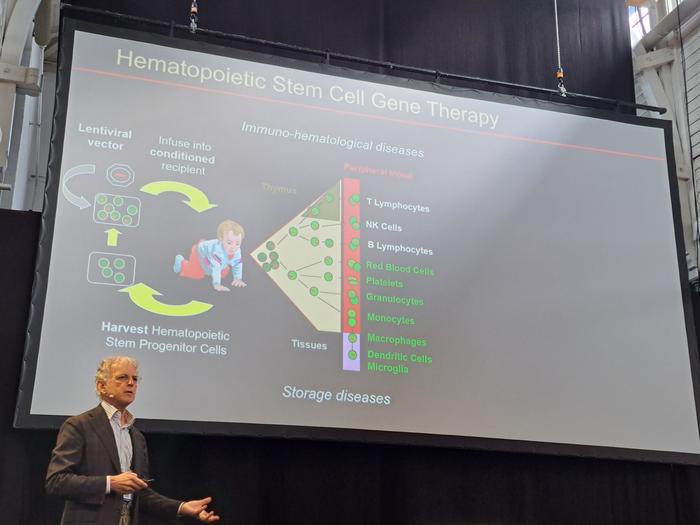
Keynote Speaker Professor Luigi Naldini
We were honoured to welcome our keynote speaker, Professor Luigi Naldini, to the stage on Tuesday afternoon, where he gave an incredibly insightful overview of the gene-editing field and in particular his work in developing gene- and gene-editing therapies for haematopoeitic disease.
It was incredible to see how far the field has come, where it stands now, and what needs to happen next.
Panel Discussion: Safety and Regulations
CMNs' science writer Rebecca Roberts did a fantastic job of moderating a lively panel discussion on Wednesday morning about standards and regulations.
Many thanks to our panelists, Samantha Maragh (National Institute of Standards & Technology, USA), Attila Sebe, MD, PhD (Paul-Ehrlich-Institute, Federal Institute for Vaccines and Biomedicines, Division of Haematology, Cell and Gene Therapy, Germany), Lotte Dahl Nissen, MSc, Ph.D (Danish Medicines Agency, DKMA), and Veronika Jekerle, PhD (European Medicines Agency, EMA).
The need for CRISPR-specific guidance and regulations, lessons learned from the recent approval of CASGEVY, international harmonisation within gene editing, and ensuring safety of gene-editing medicines for very small patient groups (e.g. for ultra-rare genetic diseases) were among the key topics discussed.

Workshops
Our hands-on workshops were held Wednesday and Thursday morning, in which more than 100 delegates took part in discussing and sharing ideas about how to choose the right gene-editing tool for a given project, delivery approaches and challenges, pre-clinical safety analysis, and translating a gene-editing programme from the lab to the clinic.
Thanks to all our wonderful faciliators for making these workshops possible.
+60 Oral Presentations and +100 Posters
Over the course of the conference, more than 160 scientists from 37 countries presented their work to an audience of 400 delegates, encompassing cutting-edge advances in gene editing, CRISPR screening, delivery, safety and clinical trials.
Rare diseases, cancer, infectious disease, neurological diseases, safety and off-targets, delivery, tools, functional genomics and regulations were among the themes in focus during the oral and poster sessions.

Poster Prizes
We thank our oral and poster presenters, and congratulate our 8 poster winners who won a certificate and free registration to CRISPRMED25:
- Rodrigo Coronel Tellez, Postdoc at Danish Technical University: Efficient sortase-mediated assembly of CRISPR-Cas9
- Colette Rogers, Postdoc at University of Minnesota: In Vivo Correction of a Genetically Humanized Fanconi Anemia Mouse Model Using Digital Editing Technologies
- Helena Escobar, Postdoc at Max-Delbrück Center - Charite, Berlin: Gene-edited primary muscle stem cells rescue dysferlin-deficient muscular dystrophy
- Federica Zinghirino, Postdoc at University College London: Development of a CRISPR-based epigenome editing platform to enhance the long-term engraftment of ex-vivo genetically modified HSPCs
- Issa Ismail, PhD fellow at Aalborg University, Denmark: Genome-wide CRISPR-Cas9 knockout screen identifies DNA damage response pathways and BTK as essential for cisplatin response in diffuse large B-cell lymphoma
- Laura Castilla-Vallmanya, Postdoc at Lund Stem Cell Centre, Sweden: Studying the role of TRIM28 and transposable elements dysregulation in neurodevelopmental disorders using CRISPRed in vitro models
- Roberta Vacca, PhD student at San Raffaele Telethon Institute for Gene Therapy, Italy: p38 MAPK fuels proliferation stress and DNA damage impairing the functionality of genetically engineered hematopoietic stem and progenitor cells
- Pietro De Angeli, Scientist, University Hospital Tübingen, Germany: EDSpliCE: A Novel Gene Editing Platform Holding Therapeutic Potential for Splicing Modulation in Inherited Retinal Disorders
Social and Networking
All work and no play makes Jack a dull boy, or so they say! Besides the fantastic scientific discussions, CRISPRMED offered a unique opportunity for researchers, clinicians and regulators in the CRISPR Medicine community to come together, make new connections and be inspired. We finished off the conference Thursday afternoon with a walk and channel tour around the beautiful city of Copenhagen, and for anyone at the front of the crowd, CMN's very own science writer and editor Gorm Palmgren took on the role of tour guide!
On behalf of the entire CRISPR Medicine News team, thanks for an amazing inaugural CRISPRMED conference. Watch this space - we will be back with a bang next year!


To get more CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Cover photo courtesy of Clarissa Braun.
Tags
CLINICAL TRIALS
Sponsors:
Suzhou Maximum Bio-tech Co., Ltd.
Sponsors:
Zhejiang University