Explainer: The Gene Drive Technology
How are gene drives generated?
Naturally occurring gene drives are found in almost all animals, including humans, plants, fungi and bacteria. They are selfish genetic elements that spread through inheritance from one generation to another. For example, homing endonuclease genes (HEGs) are site-specific selfish genes that spread by cleaving a homologous wild-type chromosome and copying themselves into the cut site through homology-directed repair (HDR). Meiotic drive is an intragenomic mechanism that interferes with meiotic processes so that the transmission of one or more alleles is favoured over another. Manipulation of transposable elements, i.e., small DNA sequences that can move from one location in the genome to another, can also generate gene drives.
Earlier attempts to generate synthetic gene drives involved gene-editing techniques such as zinc-finger nucleases (ZFNs) and transcription activator-like effector nucleases (TALENs). However, mutations occurring in the repetitive elements limited the use of these technologies for the generation of gene drives, as they were often inactivated before being passed on to the next generation.
How do CRISPR-Cas9 gene drives work?
CRISPR-Cas9 gene drives consist of a drive allele, i.e., a genetic construct carrying genes encoding the desired trait, Cas9, and a guide RNA (gRNA), as well as flanking arms homologous to the sequences surrounding the target site in the wild type chromosome.
When a homozygous gene drive-modified individual mates with an unmodified individual, the resulting offspring inherits one drive allele from the gene drive-modified parent and one non-modified version of the corresponding allele from the wild-type parent. The gRNA and the Cas9 genes are subsequently expressed in the cell, and the gRNA guides Cas9 to make a double-strand break in the wild-type chromosome at the target site. The drive allele then acts as a template for HDR, and the entire drive allele - including the gene of interest - is inserted into the wild-type chromosome. The cell is now homozygous for the drive allele, which will be passed to all gametes after meiosis, thus spreading the desired trait in the population.
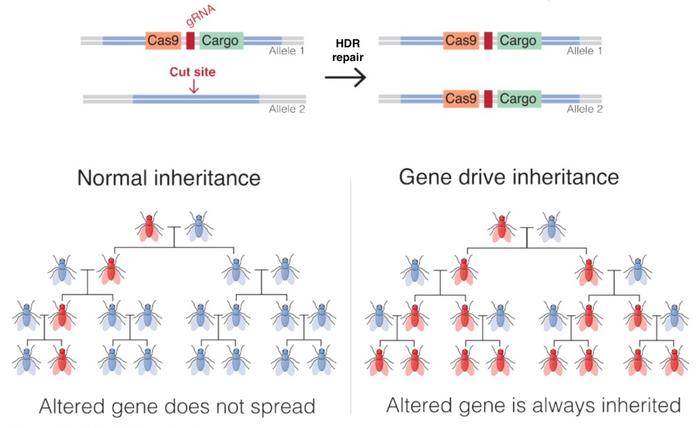
Suppression drives and modification drives are two main strategies for implementing gene drives. The idea behind suppression drives is to incorporate deleterious traits that lead to elimination of a population. Modification drives are used to disrupt or alter an undesired trait that, for example, causes resistance or allows for transmission of a pathogen, thereby leading to a less harmful population.
Applications of gene drives
Gene drives can be applied to prevent the spread of insect-borne diseases, such as malaria, dengue fever, Lyme disease, and Zika virus, by targeting the insect vector. In addition, researchers can use the technology to control other species such as rodents or fungi that transmit or cause diseases, and to combat drug resistance in pathogens. In agriculture, gene drives can be used to eliminate herbicide- and pesticide-resistance genes and thereby increase the yield of crops. Moreover, invasive species can potentially be controlled by this technology.
Limitations of gene drive technology
Despite all the benefits that gene drive technology has to offer, there are some limitations. As it has the potential to override the process of natural evolution, the technology can enforce undesired harmful genetic changes upon organisms. This can have vast and unforeseen effects on the whole ecosystem and might lead to biodiversity loss. Moreover, some scientists worry that gene drives might behave differently upon release in the ecosystem than in more restricted testing environments. These concerns are emphasised by the fact that the process is inherently irreversible.
The way forward
So far, there have been no field tests or environmental release of gene-drive modified organisms. However, a tool for controlling malaria-transmitting mosquitoes is on track for regulatory approval by 2025.
The potentially severe consequences of gene drive technology calls for action to prevent adverse outcomes. For example, researchers must develop mechanisms that allow for containment of the modified organisms, ensure that the selected traits are passed only up to a certain level, or incorporate an emergency brake that can stop further transmission of the trait. Moreover, regulatory decisions must be taken globally, and international laws are required.
Prapti Shah is a freelance writer based in Germany.
To get more of the CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
CLINICAL TRIALS
Sponsors:
Suzhou Maximum Bio-tech Co., Ltd.
Sponsors:
Zhejiang University







