FluidFM nano-injection overcomes delivery limitations of current CRISPR gene editing methods, accelerates cell line development cycles, and is poised to significantly broaden multiplexing capabilities
CRISPR’s technological breakthrough
Since its discovery and development as a gene editing technology, CRISPR has revolutionized many life science fields. It offers scientists a highly versatile gene engineering tool for a broad range of organisms and applications. The interest of the scientific community towards the use of multiplexed strategies for multi-locus editing is rising strongly: the use of multiple gRNAs can strongly enhance CRISPR’s applications, such as complex multigenic disorders modeling, de-extinction projects, or genome writing.
The delivery of multiple gRNAs into cells with traditional transfection methods is not trivial. On top of the DNA damage response caused by several DNA double strand breaks, cell viability might be impaired by stress and toxicity caused by the indirect delivery of the necessary compounds into the nucleus. All this considerably limits the potential and efficiency of CRISPR multiplexing.
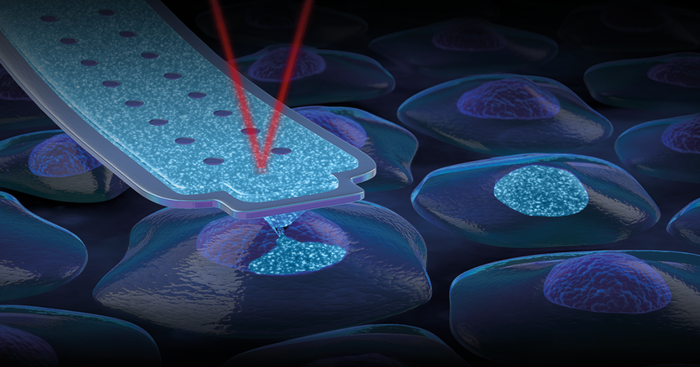
The patented FluidFM nanosyringe gently delivers the compounds directly into the nucleus of any cell (figure 1). Therefore, all the co-located reagents have the optimum stoichiometry, maximizing efficiency and eliminating vector-driven stress, while equally reducing off-target effects. By bypassing the hurdles of conventional delivery methods, CRISPR RNP complexes can be potentially co-injected with tens or even hundreds of different gRNAs. Furthermore, the direct intra-nuclear delivery and cargo-independent properties of FluidFM nano-injection make it particularly attractive when working with hard-to-transfect cells (e.g., primary cells) or when large insertions or deletions are required.
In conventional cell line development pipelines, to obtain stable monoclonal cell lines, several candidates are evaluated within an iterative process. This currently requires 12 to 14 weeks. In comparison, by favoring a “bottom-up” approach, FluidFM technology can pick a single cell that it has modified and generate a clone out of it – in less than three weeks starting from the day of transfection until the clones have been characterized.
Watch CRISPR Gene Editing Webinar
Generating multiple Knock-Out clones
Here we show how monoclonal multiple Knock-Out cell lines were generated in less than 3 weeks using FluidFM technology (figure 2). First, the genomic loci of several distinct genes were simultaneously targeted by a multiplexed FluidFM nano-injection into CHO cells, directly delivering gRNA/Cas9 RNP complexes into the nucleus. After nano-injection, the position of each transfected cell was recorded, allowing the isolation of the successfully transfected cells using a FluidFM micropipette 24 hours post-injection. Those cells were then expanded into monoclonal cultures. The sanger sequence of each cell culture was analyzed with the ICE tool to determine the success of the gene editing.
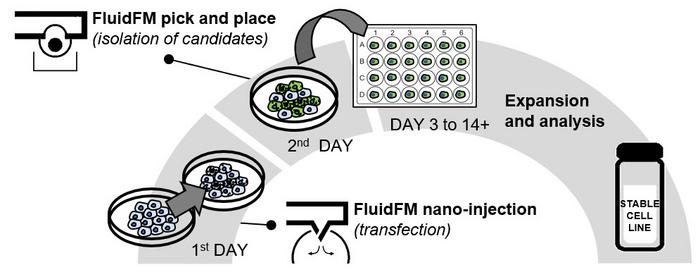
Figure 2: FluidFM Cell Line Development workflow. Day 1, cells are transfected via FluidFM nano-injection. On day 2, successfully transfected cells are selected and isolated via FluidFM pick and place. From day 3 to 14+, isolated single cells expand into a stable monoclonal cell line and their genome is analyzed. Credit: Cytosurge.
Day 1: FluidFM nano-injection
With FluidFM nano-injection, the nucleus of a few dozen CHO cells was targeted by simple point-and-click, leading to the automatic injection into the selected cells at a rate of around 5 cells/minute.
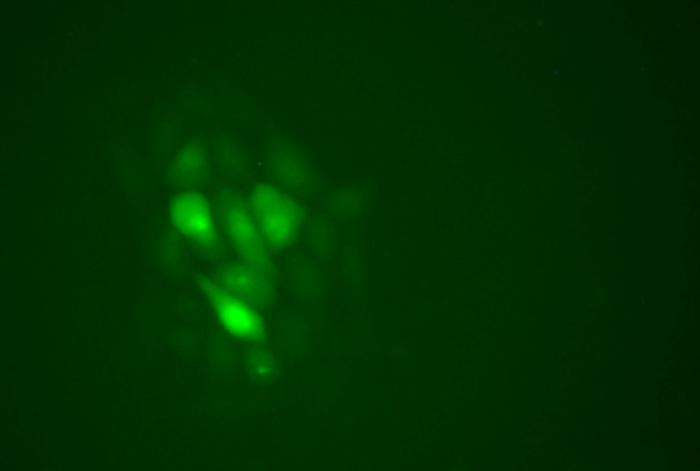
A fluorescent marker (Lucifer Yellow) was co-injected with all the different gRNA/Cas9 RNP complexes to facilitate monitoring of the injection process and to identify the best candidates (figure 3).
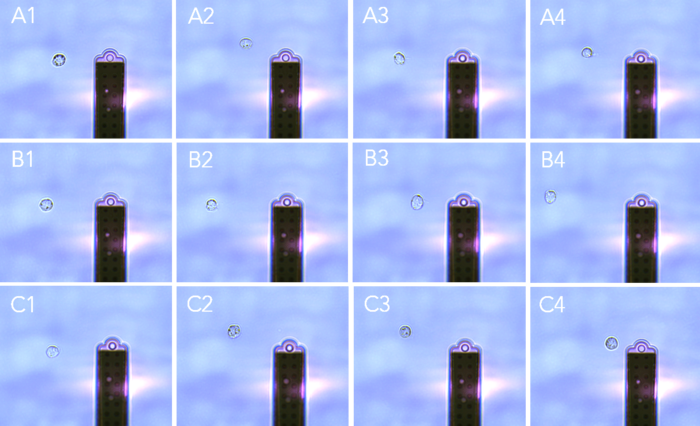
Day 2: FluidFM pick and place
24 hours after transfection by nano-injection, the targeted cells were found again using the integrated FluidFM BIO Series operator software (ARYA). Candidates were isolated with a FluidFM micropipette with a 4 μm aperture and were placed into an empty well (figure 4). Monoclonality is guaranteed by visual confirmation.
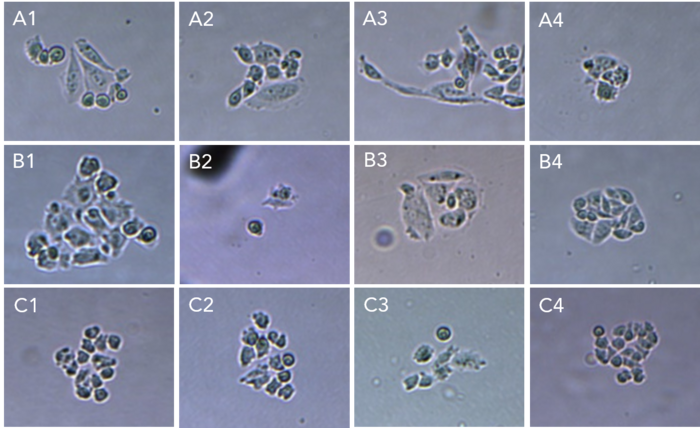
Day 3 – 14: Culture expansion and indels analysis
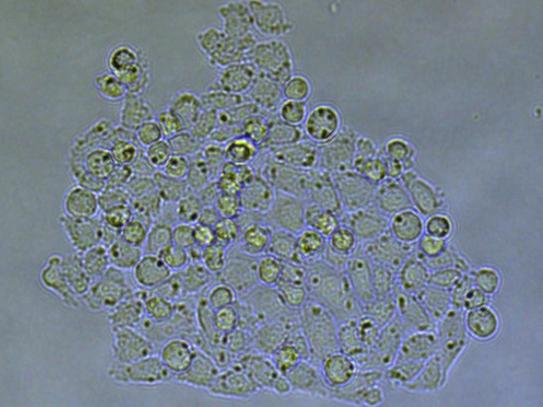
The clones were incubated after isolation and their growth monitored after 3 and 6 days (figures 5.1 and 5.2). More than 90% of the isolated cells developed into a colony. 14 days after transfection, the clones were collected and analyzed for all the targeted genes using Sanger sequencing. Overall, 50% of the clones showed mutations in targeted loci.
Conclusion
The presented results show that multiple gRNAs can be simultaneously delivered into selected single cells by FluidFM nano- injection. We believe that CRISPR multiplexing with the presented method can be scaled up to simultaneously co-inject several tens of gRNAs. Furthermore, we prove here that the FluidFM “bottom-up” approach drastically reduces the development time for monoclonal cell lines with multiple desired phenotypes from months to just a few weeks.
Outlook
The technological advances brought by FluidFM into the field of single-cell gene engineering have the potential to solve some of the most demanding challenges that scientists are currently facing when they need to rapidly and efficiently develop monoclonal cell lines. Current methods are adequate when applied to the most common cell lines and gene engineering strategies. However, they quickly reach their limits when dealing with uncommon, rare or fragile primary cell types that are also known to be hard-to-transfect, or when complex experimental design - for example, CRISPR multiplexing - is needed. In these particular cases, FluidFM may be the only available solution.
More on FluidFM technology and CRISPR editing
Jennifer Rottenberger, Justin S Antony and Markus Mezger are afilliated to University Children's Hospital, Department of Pediatrics I, Hematology and Oncology, University of Tübingen, Tübingen, Germany.
Paul Monnier, Maria Milla, Tobias Beyer and Dario Ossola are afilliated to Cytosurge AG, Saegereistrasse 25, 8152 Glattbrugg, Switzerland.
Sponsored content from Cytosurge AG. Cytosurge AG develops, manufactures and distributes state-of-the-art nanotechnology solutions and systems based on its patented FluidFM® technology. Copyright © 2020 Cytosurge AG, Switzerland. All rights reserved.
Tags
ArticleSponsored ContentDeliveryNon-viralMicroinjectionQuality ControlCRISPR-CasCytosurge AG
CLINICAL TRIALS
Sponsors:
Suzhou Maximum Bio-tech Co., Ltd.
Sponsors:
Zhejiang University







