Using CRISPR to Improve CAR-T Cells
Welcome back to Making the Cut! Last week we continued our miniseries about CAR-T cells and told you everything you needed to know about how we can use CRISPR to make off-the-shelf CAR-T cells that can be readily used to treat a patient in need. But CRISPR has the potential to bring CAR-T therapy even a step further. So, today we’re going to tell you how we can CRISPR to make CAR-T cells even better at fighting cancer.
But before a quick disclosure: we are not immunologists. You got it by now, but for clarity’ sake, we are mainly CRISPR guys. As such, consider this as the tip of the iceberg, there is a whole world about Immunotherapy and we are here just to spark your interest and encourage you to learn more about it!
Exhausted: the never-ending battle
We know what you’re thinking: “I thought CAR-T cells were super soldiers that destroyed cancer. Do we need to make them better?” It may surprise you, but the answer is yes.
Think about your favourite superhero, whether it’s Captain America, Spiderman, or Batman. What do they all have in common? Apart from being badass and wearing lycra outfits, that is.
As powerful as they are, and as efficiently as they can kick the asses of the bad guys, all superheroes have a limit. Of course, Batman can take on 10 guys at the same time, but give him a hundred and he may eventually get a bit tired.

That’s exactly what can happen to our CAR-T cells. They can get exhausted. And yes, that is a scientific term, believe it or not. But what does it mean when a CAR-T cell gets exhausted? Well, if Batman got exhausted, his punches would probably get weaker, and he would maybe let some bad guys escape, right? The same thing happens with CAR-Ts. When they get exhausted, they are less effective at killing cancer cells, which does not bode well for the patient, does it?

Interestingly, scientists have been able to figure out which mechanisms lead to cell exhaustion, and which changes they cause in the CAR-T cell. And when we understand how something works, CRISPR allows us to engineer it!
Sucker punch: CRISPR-mediated inhibition of PD1 avoids cell exhaustion
So, what is this mysterious mechanism for CAR-T exhaustion? While CAR-T exhaustion results from the interplay of several stimuli, the main problem is that the CAR-T cell, by finding enemies one after the other, is stimulated all the time. And scientists discovered that one of the key elements for this process is a protein called PD1. This cellular receptor binds to ligands called PDL1 and PDL2, which produces a reduction in the activity and effectiveness of the CAR-T. And these ligands are all over the place in the tumor and its environment. So, the longer the CAR-T cell is there, the more exhausted it’ll get.
PD1 and other receptors are part of the so-called Immune checkpoint inhibitor cascade. Remember when we told you that our immune system can be over-protective? This is another vivid example. See, PD1 – and its peers – aren’t there just to mess with our therapy, but in principle, they prevent T cells from getting too trigger-happy. In fact, if an infection – or cancer – goes on for a long time, all the cytokines and other effector molecules released by the T cells may also damage healthy tissues. So, PD1 & Co. act as a “safety break”. This mechanism unfortunately backfires when cancer cells take advantage of it to underpower T cells.
So, in this set-up you can think of PD1 as the devil on the CAR-T’s shoulder, telling it “This is too much, you’re so tired, you should take a break”. So, what can we do with this devil? Thankfully, we have an angel called CRISPR which can sucker-punch it and get it, and its negative thoughts, out of the way. Indeed, we can use CRISPR to knock out PD1, which makes CAR-T cells way more resistant to exhaustion, increasing their effectiveness in killing cancer cells.
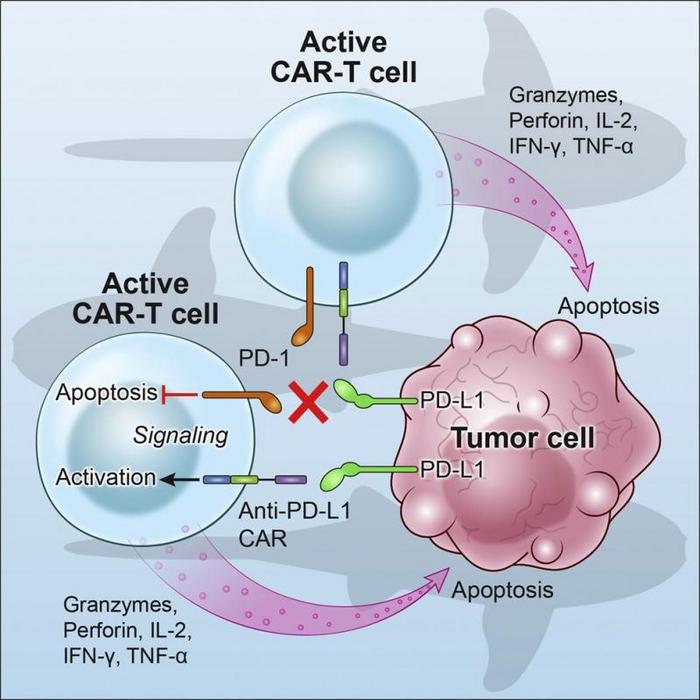
Apart from PD1 other, less relevant little devils can do similar functions, such as TIM3 and LAG3. Also in these cases, knocking them out improves CAR-T activity. Punch after punch, CRISPR helps CAR-Ts focus on the task at hand, destroying the tumor!
The tumor fights back: how the tumor microenvironment can defeat CAR-T cells
Okay, so now we have super soldiers with an iron will, who won’t let anything wear them down… right? Well, yes and no. You see, like in every superhero movie, CAR-Ts must face off against a very strong and cunning villain, the tumor. Now you may think of a tumor as a clump of cancerous cells, but it is way more complicated than that.
Indeed, the tumor has its own microenvironment, which is kind of like the villain’s lair. And it is full of traps that can make our brave CAR-T cells fail their mission. “And what are those traps?”, we hear you asking. The main trap are immunosuppressive cytokines that tumor cells can secrete. These cytokines are like arrows that try to bring down our CAR-Ts before they can even reach the tumor. Good thing for us, differently to arrows, cytokines need to bind a surface receptor to have their effect. And what have we learned already? Yes, we can CRISPR the hell out of that receptor and knock it out!

In particular, knockout of the receptor for TGF-β avoids the immunosuppressive effects of this cytokine, ensuring the high activity of our CAR-Ts. And, as before, other receptors involved in this pathway can also be knocked out. This is an area of research that is rapidly expanding, and hopefully in the next years scientists will be able to develop CAR-Ts without any weakness.
So, there you have it! As we promised! Now you know how we can use CRISPR to superpower our CAR-Ts so that they become the best version of the superhero they were always meant to be!
But wait, there’s more! Because CRISPR is not done being awesome, and we’re not done telling you about it. Next week, we’ll look at how CRISPR technology can be used to make CAR-T cells in the first place. Because of course it can! Until next time!
To get more CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
CLINICAL TRIALS
Sponsors:
Suzhou Maximum Bio-tech Co., Ltd.
Sponsors:
Zhejiang University