A GEENIE in a Bottle: CyGenica’s Unique Non-Viral Cargo Delivery Platform

Hi Nusrat, I’m looking forward to learning more about CyGenica’s delivery technology, but before we get into all of that, could you tell us about the company’s origins and how it came to life?
CyGenica was founded in 2017, but the mission that started it all actually goes back to 2000. I was doing my PhD in chemistry in India when my father became sick with cancer and the available treatments were highly toxic. As a chemist, I set out to synthesise new versions of those anti-cancer drugs that would be safer. I produced many compounds that showed excellent anti-cancer activities in vitro, but unfortunately they turned out to be very toxic in animal studies.
By then, 5 years had passed and I didn’t want to spend more time synthesising new molecules. Instead, I wanted to understand more about the biological mechanisms of action of these compounds. I switched from chemistry to biology and spent some years studying protein structure and function in various institutes around the world, but it wasn’t until I joined Professor Takafumi Ueno’s laboratory as a researcher in the Institute of Integrated Cell Material Science (iCeMS) at Kyoto University in Japan that I serendipitously found a protein that would eventually inspire CyGenica’s proprietary delivery platform.
And how did this discovery come about?
Professor Ueno had invited me to join his lab in iCeMS, the establishment of which was part of a national Japanese interdisciplinary initiative to ignite new discoveries by having biologists do chemistry, chemists do biology, physicists do chemistry, and so on. The idea was that a scientist coming from another discipline would approach a problem differently, and that really triggered my curiosity.
By this stage, I knew how to synthesise compounds and I had a good understanding of proteins and how they fold. But in Kyoto I got the unique chance to investigate exactly what happens when drugs/molecules are added to cells, and I found a protein with a very intriguing structure that entered into living cells.
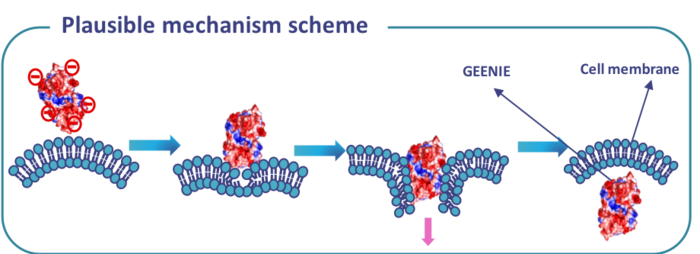
A molecular drill that bores through the cell membrane
So CyGenica started because of a single protein? What was so special about this protein?
This protein could go through living cells by a unique non-endocytic helical mechanism, which completely defied the known mechanisms of cell penetration. Cell penetration usually occurs through the endocytic pathway whereby positively-charged molecules are designed to be cell-penetrating. But this protein was a negatively-charged molecule that was still going through the cells, and that caught my attention.
I hypothesised based on that discovery that it should be possible to make almost any molecule cell-penetrating. This idea brought me back to India in 2016, where I was awarded an Indian grant to try and prove my hypothesis. Within a year, I could prove it - if I designed a molecule based on the hypothesised criteria, it became cell-penetrating!
Around this time, I was accepted into the RebelBio accelerator programme in Ireland, and the company received accelerator funding followed by pre-seed funding from SOS Ventures in Ireland as well as several grants from Indian agencies.
You mentioned that the protein you found in Kyoto would inspire CyGenica’s GEENIE platform. Can you tell us more about what GEENIE looks like today?
First of all, GEENIE stands for ‘enablinG safE and targEted iNtracellular delIvEry’. We envision that GEENIE will be used to deliver therapeutics for rare genetic diseases, cancer, and infectious diseases.
GEENIE can be thought of as a protein drill that bores through the cell membrane and brings its cargo along with it. It is a 48-KDa protein that is taken into cells by a non-endocytic mechanism that doesn’t damage the cells. It delivers its cargoes to the nucleus, which is highly relevant for gene-editing therapeutics.
Another attractive feature of the platform is that we can easily control how fast the cargo is released from GEENIE once inside the cell by influencing the chemistry, i.e., by carefully choosing an appropriate linker to fuse GEENIE to the cargo. Some linkers will be broken down quite fast inside the cell, while others will persist for longer, resulting in a slower release of the cargo.
The protein that I found in Kyoto is not the protein behind GEENIE. Based on the structure and features of the protein I found, we found another similar protein elsewhere, and engineered it until it became what we now call GEENIE. To use the platform, we make a fusion between the GEENIE protein and its intended cargo, which could be CRISPR reagents, an anti-cancer drug or even an antibiotic.
CyGenica is an Irish-Indian company founded in 2017 that specialises in novel cargo delivery technology. The company has developed a non-viral, non-toxic platform called GEENIE to develop in vitro and in vivo delivery solutions for drugs, gene editing components, and antibiotics. For more details see the company's website here.
See and hear Dr Nusrat Sanghamitra present CyGenica's novel delivery platform at upcoming free CMN webinar on the 26th October 2022. See here for details.
How does GEENIE compare to other gene-editing delivery vehicles in terms of capacity?
We can deliver the early Cas9s, which are 180KDa in size, without any problems apart from delivery being slowed slightly. We’ve also tested plasmids up to 2.4 Kb in size and we could deliver those without any issues. Fortunately, because we are conjugating GEENIE to its cargo, once GEENIE gets into the cell the cargo is there too. In this way, we don’t lose cargo.
Organ-specific targeting
So the idea is that GEENIE can be used to deliver a wide range of diverse therapeutics to cells. How does that work in practice, and is it necessary to modify GEENIE each time it is used for a different cargo?
To deliver organic molecules, we don't engineer GEENIE in any way. We simply use the appropriate chemical reaction to fuse GEENIE to whatever small molecule we want to deliver.
To fuse GEENIE with biological cargoes, such as protein, there are two options depending on the cargo in question. The first is chemical fusion, which is similar to what I just described for organic molecules, and involves using chemical linkers to conjugate GEENIE to its cargo. However, for genetic fusion, for instance to deliver CRISPR reagents via plasmid DNA, we would need to create a construct to conjugate GEENIE to the plasmid. This is done on a case-by-case basis. We’ve shown that this works for CRISPR-Cas9 gene editing, where we can chemically or genetically conjugate GEENIE to a Cas9 protein, and then chemically conjugate the same or another version of GEENIE to a guide RNA.
What about targeting different organs? I understand that CyGenica has already developed targeted delivery strategies for certain cancers. How does this work?
For each target organ, we engineer GEENIE separately to make it ‘go’ to the right place in the body. We have so far proven that our strategy works in vitro in HER2+ breast cancer and in glioblastoma. For each of these targets, we have developed a specific construct that recognises a unique receptor on the target cells: this is HER2 in HER2+ breast cancer and an undisclosed receptor in glioblastoma.
We believe that our strategy can be applied to any tissue type as long as a unique receptor has been identified. We are currently working on targeting strategies for the lung, where we already have a receptor, and the kidney for which I’m still searching for a receptor.
As an interesting side note, we have so far administered GEENIE to animals as an intravenous injection, but because this protein is so stable, we may try to develop an inhalation method for lung treatments in the future.
Multi-organ delivery with hints of blood brain barrier penetration
You said that GEENIE should enter the target organ via a specific receptor. How precise is this, or in other words where does GEENIE end up when it’s administered in vivo?
We performed biodistribution studies in mice that were injected intravenously with GEENIE, and found that GEENIE has a wide and good distribution profile across all organs. In fact, compared to some of the other non-viral delivery vehicles such as liposomes and cell-penetrating peptides, where the cargo is often gone after 30 minutes to 1 hour, GEENIE’s 24- to 48-hour distribution profile is very good, so we are very keen to explore its use in many different organ types.
We have also seen indications from these studies that GEENIE goes to the brain, which is a very exciting prospect given the massive bottleneck in getting drugs across the blood-brain barrier. We’re currently investigating this possibility in detailed animal studies of glioblastoma including brain dissection studies.
Delivery systems for gene editors
The most crucial roadblock to intracellular delivery of large molecules, especially for gene therapy, remains the cellular membrane. This biological barrier is a double-edged sword; while it prevents infectious agents from entering the body and causing disease, it also hampers the successful use of life-saving drugs and advanced therapeutic technologies.
Within gene editing, there are currently three key approaches to delivery – viral, chemical, and physical – each of which comprises several different vehicles. Viral delivery vehicles include adeno-associated viruses (AAVs), full-size adenoviruses (AdVs), and lentiviruses (LVs). Chemical delivery vehicles, otherwise known as nano delivery vehicles, include liposomes and lipid nanoparticles, lipoplexes and polyplexes, inorganic nanoparticles, and cell-penetrating peptides. Physical delivery vehicles include electroporation, microinjection, microfluidics, and hydrodynamic delivery.
The vehicle chosen for delivery must be compatible with several other key factors to achieve an efficient and safe therapeutic outcome. Within therapeutic gene editing, these factors include the gene-editing cargo used, whether it be a DNA plasmid, mRNA, or a ribonucleoprotein complex, the nature of the experiment i.e. in vivo, ex vivo, or in vitro, and the target tissue. The successful delivery of therapeutics into target cells and organelles would minimise the occurrence of undesired ‘off target’ side effects and reduce the required dose.
Strong safety profile and no immunogenicity
Within gene editing, just a few in vivo programmes have advanced, mainly for the liver and the eye. For other organs, it seems to be a challenge to deliver the gene-editing cargo in high enough quantities to achieve efficient editing at a safe dosing level. How safe is GEENIE?
We have shown that GEENIE is non-toxic even at an extremely high dose of 1,800 mg per kg in mice. To put that into perspective, the FDA recommends 2,000 mg per kg as a maximum dose in single-dose safety studies. We tested at 1,800 mg because of a material shortage and found no signs of toxicity or immunogenicity whatsoever in vivo in mice.
In ex vivo studies, we observed a very statistically insignificant cytokine response. The lack of immunogenicity sets GEENIE apart from viral delivery methods such as the adeno-associated viral vectors and lentiviral vectors that are often used in gene therapies. Beyond the safety advantage, as a simple protein, GEENIE is also much easier to work with and more cost-effective than therapeutic-grade viral culture.
You mentioned that GEENIE has a good distribution profile after 24 to 48 hours? In terms of safety, for how long does it ‘hang around’ in cells? For gene editing, it won’t be needed once the editing is done.
Since GEENIE is a protein, it will automatically be degraded by proteases inside the cell. We don’t know exactly how many days this takes but we can see from our biodistribution studies in mice that there is very little GEENIE present after 48 hours.
Giving new life to old antibiotics
We focused mainly on gene-editing therapeutics today, but I also want to touch upon a feature of GEENIE that I find very intriguing. I read that it it may help to overcome acquired antibiotic resistance. How would this work?
We’ve seen that GEENIE can cross the cell membrane barrier of multi-drug resistant bacteria and carry fluorescent dyes into the cells. When it comes to antibiotic resistance, there are two main mechanisms. The first is that the drugs do not penetrate the bacterial cell membrane at all, and the second is that the drugs penetrate but are pumped back out again by protein-based efflux pumps located at the bacterial cell membrane.
We haven’t gone into this area heavily yet, but we see potential for using GEENIE here in two ways. By conjugating GEENIE to a non-penetrable drug, we may be able to deliver the drug inside resistant bacterial cells. In addition, conjugating an antibiotic with GEENIE will render the resulting complex far too large to be effluxed by the pumps. If we can conjugate antibiotics to GEENIE without altering their modes of action, we may be able to revive the antibiotics that have become redundant because of widespread drug resistance.
Partnerships with drug developers
As a start-up company with a unique platform that has potential applications across many areas, what is the long-term vision for CyGenica? Will the company eventually develop its own therapeutic pipeline?
We envision proving GEENIE as a platform by building a pipeline of candidates that we will advance to a certain extent. For instance, we are now working on early pre-clinical studies for glioblastoma and HER2+ breast cancer, using GEENIE to deliver chemotherapeutic drugs, and we hope to take one of these to a first-in-human trial in 2025. At the same time, we are actively looking for partners to engage in R & D partnerships, co-development partnership, as well as partners for out-licensing agreements.
What does CyGenica need to do to get to a first-in-human trial by 2025?
We need to compile a package of data from IND-enabling studies before we can apply to the FDA to start a clinical trial. We have already performed many of these studies sporadically with encouraging results, for instance the MTD (maximum tolerated dose) level and the single-dose toxicity study, so we are confident that we can provide the full package within 2 years, once we have proof-of-concept that GEENIE works in animals. But, of course this all depends on funding.
This has been really interesting, Nusrat. Thanks for the chat and I look forward to seeing what happens next for CyGenica!
To get more of the CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
CLINICAL TRIALS
Sponsors:
Suzhou Maximum Bio-tech Co., Ltd.
Sponsors:
Zhejiang University







