A New ‘MILESTONE’: CRISPR-Based Approach Tackles Genetic Cause of Congenital Neutropenia

Severe congenital neutropenia (CN) is a genetic bone marrow failure syndrome characterised by a lack of mature neutrophils, rendering individuals susceptible to life-threatening bacterial infections from birth. The disease is commonly caused by autosomal dominant ELANE mutations. Current treatments have several limitations, including a high risk of complications.
To address the unmet need for curative therapies, researchers at University Hospital Tübingen and the University of Freiburg developed an innovative gene-editing therapeutic approach that could provide safe and effective treatment for CN. The proposed approach involves the use of CRISPR/Cas9 nickases to target the TATA box of the ELANE promoter and inhibit ELANE mRNA expression without modifying the ELANE coding sequence.
This approach restored granulocytic differentiation of ELANE-CN CD34+ haematopoietic stem and progenitor cells (HSPCs) both in vitro and in vivo without affecting the function of edited neutrophils. The authors propose that CRISPR-based gene editing of ELANE-CN HSPCs could provide a universal, safe, and curative therapy for patients with ELANE-related CN.
»Our study investigated a universal gene therapy approach for patients with CN who carry autosomal dominant mutations in the ELANE gene, which represents the largest group of CN patients,« said Masoud Nasri, PhD, the study's co-first and corresponding author. »This approach provides a curative therapy that eliminates G-CSF dependence, minimises the risk of leukaemia development, and reduces the complications associated with allogeneic stem cell transplantation.«
The study was published in Molecular Therapy.
Rationale: Addressing the genetic cause of congenital neutropenia
Autosomal dominant mutations in the ELANE gene, which encodes the neutrophil elastase protein, are responsible for bone marrow failure in approximately half of patients with CN. These mutations disrupt the proper development and function of neutrophils, leading to recurrent and severe bacterial infections that often start in infancy.
Current treatments, such as daily subcutaneous injections of G-CSF, can help boost neutrophil production; however, they do not address the underlying genetic cause of the disease. »This therapy increases the neutrophil count to prevent severe life-threatening bacterial infections, but it does not cure the disease,« explained Dr. Nasri.
Moreover, some patients do not respond to G-CSF, and the treatment can have significant side effects, especially as patients grow older. In addition, G-CSF injections do not prevent the development of leukaemia, which occurs in approximately 15% of patients with CN. The only potentially curative option, allogeneic stem cell transplantation, carries a high risk of serious complications, including graft-versus-host disease, graft failure, and transplant-related mortality.
Approach: Developing a CRISPR-based gene therapy to inhibit ELANE expression
To address the unmet need for new therapeutic strategies that can safely and effectively treat the genetic cause of CN, researchers developed a gene-editing approach called MILESTONE. This CRISPR/Cas9D10A-based gene-editing approach involves the use of a pair of guide RNAs that bind to opposite strands of the ELANE promoter TATA box, which is crucial for gene expression.
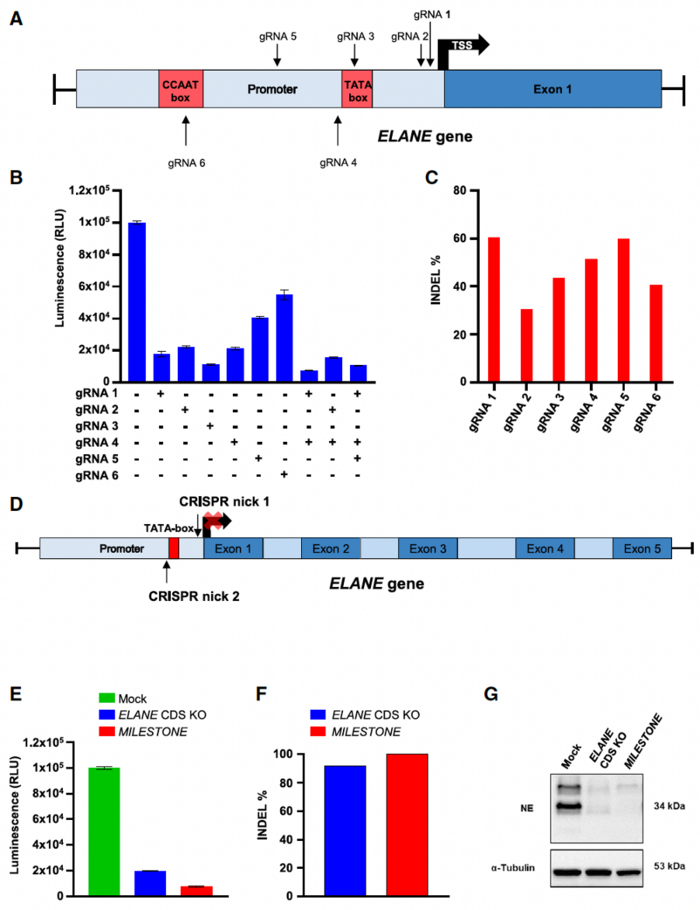
The dual-nickase strategy introduces a controlled double-strand break in the DNA, leading to small deletions that disrupt ELANE transcription without altering the gene's coding sequence (Figure 1). This approach led to a 10-fold decrease in ELANE expression 72 hours after gRNA electroporation.
Dr. Nasri explained that they had previously attempted to knock out the ELANE gene by inducing frameshift mutations in its coding region. However, potential safety issues associated with the introduction of unintended in-frame deletions and insertions (indels) in ELANE have driven them to develop an alternative strategy to target the regulatory region rather than the coding region of the gene to avoid the introduction of new ELANE variants. The team found that MILESTONE provided a >90% on-target editing frequency, comparable with on-target editing with their previous approach targeting the ELANE coding sequence.
»The long-term consequences of these unintended indels in ELANE for transplanted patients are unknown. It is especially crucial because we are dealing with pre-leukaemia bone marrow failure syndrome,« noted Dr. Nasri. »The new ELANE-targeting approach overcomes safety concerns by targeting the non-coding region of ELANE and using a dual Cas9 nickase approach.«
MILESTONE editing restores granulocytic differentiation
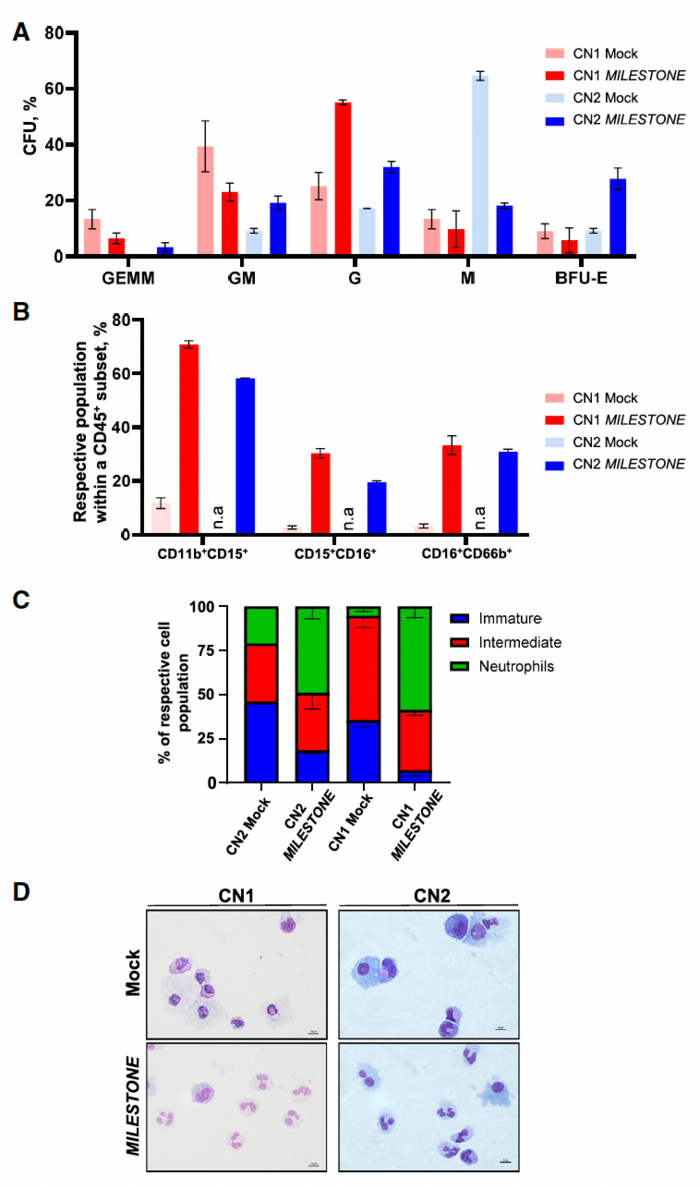
When the team applied MILESTONE editing to CD34+ HSPCs derived from two patients with ELANE-related CN, they observed remarkable restoration of defective neutrophil differentiation in vitro. The percentage of CD11b+/CD15+ leukocytes, representing mature neutrophilic granulocytes, increased from 10% to 70% for cells from patient 1 and up to 60% for cells from patient 2 (Figure 2).
»Although the precise mechanisms remain unclear, it is thought that mutant neutrophil elastase induces the unfolded protein response, endoplasmic reticulum stress and deregulates G-CSFR-triggered granulopoiesis signal transduction, leading to the maturation arrest of granulopoiesis observed in patients' HSPCs,« said Malte Ritter, co-first author of the study. »By reducing neutrophil elastase levels, MILESTONE mitigates the damaging effects of mutated neutrophil elastase, allowing the stem cells to undergo normal granulocytic differentiation.«
Neutrophil elastase is a proteinase used by neutrophils for bacterial killing and protein degradation. Therefore, the investigators next assessed whether ELANE silencing influences the function of neutrophils. Despite the reduction in ELANE mRNA, edited neutrophils from healthy donor HSPCs maintained their functional capabilities, such as phagocytosis, chemotaxis, and reactive oxygen species production.
»We plan to broaden the studies on neutrophil functional profiles and investigate all potential capabilities, such as fungicidal, NETosis, and antibacterial activities against a broad spectrum of bacteria,« said Ritter.
To assess the effect of MILESTONE editing on engraftment of ELANE-CN HSPCs in vivo, the researchers injected immune-deficient mice with edited HSPCs. Sixteen weeks after transplantation, MILESTONE-edited cells showed strong engraftment, with 100% of cells carrying indels in all recipient mice. In contrast, mock-edited cells engrafted poorly, with approximately 5% of cells carrying indels. Moreover, the percentage of neutrophils in the bone marrow of xenografted mice was markedly higher in the MILESTONE group than in the control group (0.25% versus 0%).
MILESTONE causes no significant off-target effects
Potential off-target effects are a critical safety concern for gene editing-based therapies. To assess the safety profile of the MILESTONE approach, the researchers employed advanced genome-wide off-target profiling techniques such as GUIDE-seq and CAST-seq.
These assays showed over 90% on-target efficiency with MILESTONE, with only two potential off-target sites, and no chromosomal translocations. For comparison, the previous method targeting the ELANE coding sequence had seven potential off-target sites.
To further confirm the specificity of their approach, the researchers performed RNA sequencing of MILESTONE- and mock-edited primary CD34+ HSPCs from two healthy donors. In addition to ELANE, which was significantly downregulated by more than 9-fold (log2-fold = 3.21; adjusted P-value = 2.68 x 10-7), only one other gene, PHGDH (phosphoglycerate dehydrogenase), was significantly downregulated (log2-fold change = 1.44, adjusted P-value 2.10 x 10-2).
Potential implications and future work
Although further validation is needed, the researchers envision that MILESTONE editing of autologous HSPCs could provide a universal, potentially curative treatment option for patients with ELANE-related CN, regardless of their gain-of-function mutation. This approach could possibly replace the need for lifelong G-CSF injections and reduce the risk of myelodysplastic syndrome or acute myeloid leukaemia.
»MILESTONE achieves a level of precision that is difficult to reach with other strategies due to the use of paired nickases and is a universal approach applicable as gene therapy for all ELANE-related congenital neutropenia patients,« said Dr. Nasri.
While this proof-of-concept study provides evidence to support the use of MILESTONE as a new gene-editing therapeutic strategy for ELANE-related CN, the researchers acknowledge that further studies are needed to validate their findings and assess the long-term safety and efficacy of their approach. Extending the in vivo experiments to larger animal models and monitoring for potential genotoxic effects or clonal abnormalities over extended time frames will be crucial steps before testing this approach in humans.
»Congenital neutropenia is a pre-leukaemic bone marrow failure syndrome, so we need to be sure that by editing haematopoietic cells, we are preventing rather than potentiating the development of leukaemia,« Ritter said. »Optimising HSPC transplantation regimens, developing patient selection criteria, and ethical considerations for patients, parents, clinicians, and other stakeholders are essential.«
The team also plans to investigate whether the MILESTONE strategy could be applied to other genetic disorders caused by gain-of-function mutations, where inhibiting gene expression may be preferable to correcting the coding sequence.
»This strategy can potentially be applied in a broad spectrum of autosomal dominant disorders where precise knockdown of disease-causing genes with gain-of-function mutations can yield therapeutic benefits, provided that the targeted gene has no essential function or its function can be compensated by another protein,« Dr. Nasri concluded.
Link to the original article in Molecular Therapy:
CRISPR/Cas9n-mediated ELANE promoter editing for gene therapy of severe congenital neutropenia
Christos Evangelou, PhD, is a freelance medical writer and science communications consultant.
To get more CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
CLINICAL TRIALS
Sponsors:
Suzhou Maximum Bio-tech Co., Ltd.
Sponsors:
Zhejiang University







