Appetite for Destruction: The Indiscriminate Nuclease Activity of Cas12a2

From a biochemistry perspective, characterising the activity of a new Cas nuclease seems simple enough: purify the protein, put it in a test tube with DNA and a guide for a target DNA sequence, then measure what happens. It’s this approach that Assistant Professor Ryan Jackson and his team at Utah State University took when attempting to characterise the biological activity of Cas12a2 from Sulfuricurvum sp. (SuCas12a2). But the results they achieved were, in short, baffling.
»Whenever we put DNA with Cas12a2 in the test tubes, one of two things would happen. Either nothing would happen at all, or all of the DNA would disappear. All of it, whether it was our control or a target. We kept hitting this dead end over, and over, and over,« Jackson says.
Luckily for the team, Jackson’s specialty is overcoming dead ends. After completing his PhD in structural biology, he worked in the early wave of CRISPR-Cas9 technology during his postdoctoral research. When new CRISPR systems started being discovered, Jackson knew he wanted to figure out how they function and ended up investigating obscure and little-researched CRISPR systems.
With perseverance, Jackson and his group ended up making some surprising discoveries about Cas12a2. As part of a collaboration with Professor Chase Beisel and his team at the Helmholtz Institute for RNA-based Infection Research in Germany, they recently published a duo of papers in Nature describing Cas12a2. The first details the biological activity of the nuclease, while the second delves into the structure and activation pathway of Cas12a2.
The work also involved the Agricultural Biotechnology company Benson Hill, which originally discovered the nucleases, as well as David Taylor’s group at the University of Texas, Austin, who generated the Cas12a2 structures.
»What Cas12a2 does that's so unique is the collateral destruction of double-stranded DNA (dsDNA) even though it recognises an RNA target. That is really interesting, and that's where there are probably some really cool applications,« Jackson remarks.
Population-level immunity: Cas12a2 induces abortive infection
Beisel and Jackson were originally working on the premise that Cas12a2 targets DNA – a logical assumption, given that it belongs to the type V CRISPR nucleases, which cleave dsDNA, and its closest evolutionary neighbour is the DNA-targeting Cas12a.
But Cas12a2 is a bit of a wildcard. Despite its phylogenetic placement with DNA-targeting nucleases, Cas12a2 targets and cleaves RNA. Even more interesting, however, is the fact that once it cleaves its RNA target, Cas12a2 attacks every other nucleic acid present in the cell or test tube: single-stranded RNA (ssRNA), single-stranded DNA (ssDNA), and dsDNA.
When Jackson and his team put Cas12a2 in test tubes with pure DNA and a guide, nothing would happen, because its target wasn’t present, and it remained inactive. But sometimes, despite the lab’s best efforts, a small amount of activating RNA would be present, contaminating the sample. When RNA was present and Cas12a2 recognised its target, it would go berserk, destroying all the nucleic acids in the tube.
Unlike many known Cas nucleases, which protect bacterial cells from invading bacteriophage, this nuclease has a more sinister role. Cas12a2 is an assassin, and its instructions are simple: if the target phage is present, shut down the cell. Known as abortive infection, this mechanism ensures the infected cell/s will go dormant or die before the phage can replicate and spread to the rest of the bacterial colony, achieving population-level immunity.
»We realised that we had something that was totally unlike other CRISPR systems. Instead of this CRISPR system protecting the cell, like Cas9 and Cas12a, when this Cas12a2 system finds its target, the cell dies,« Jackson comments.
Beisel was the key collaborator who asked Jackson and his team to work on the Cas12a2 project. Originally a chemical engineer, Beisel ventured into synthetic biology and now specialises in RNA engineering. His research focuses on understanding how CRISPR immune systems function and applying those insights to improve CRISPR technology. With his knowledge of CRISPR systems, he was able to contextualise the role of Cas12a2 in abortive infection.
»Abortive infection really seems to be the common approach for bacteria when they’re trying to fend off an invader. In many regards, CRISPR systems that actually clear the invader are the weird exception, rather than the rule,« Beisel explains.
A shared CRISPR array, structural oddities, and a unique activation mechanism
Despite being evolutionary neighbours, Cas12a and Cas12a2 are remarkably different, with only 10-20% sequence similarity. Yet, in many cases, Cas12a2 actually shares its CRISPR array with Cas12a. Why this is the case remains a key question.
»Every CRISPR system discovered to date has its own CRISPR array. This is the only time where you actually have two separate nucleases paired with each other. It’s extremely unusual,« Beisel remarks.
The structural paper details the purified binary complex of the catalytically active Cas12a2 with its mature CRISPR RNA (crRNA), revealing its structure to resemble an oyster – quite different to Cas12a’s ‘sea conch’ shape (Figure 1). Cas12a2 also has an α-helical recognition (REC) lobe which is completely unique – no known homologues exist.
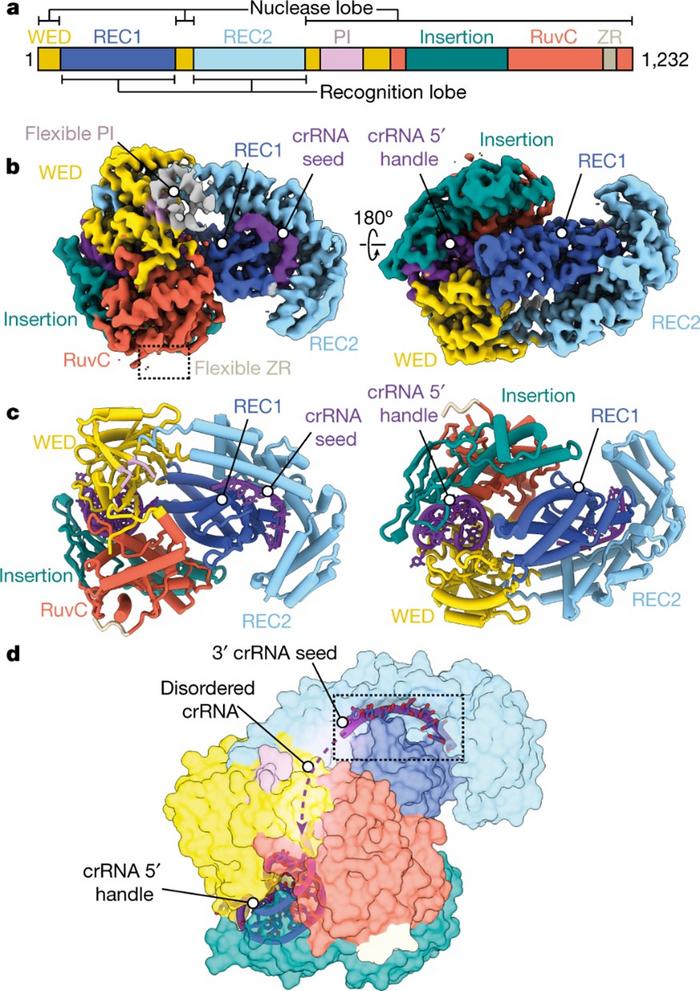
The team also revealed that the activation mechanism is distinct compared to other Cas12 nucleases. Binding to the RNA target causes significant conformational changes in Cas12a2 that allow it to unleash its indiscriminate nuclease activity.
By leaving the RuvC active site accessible in a positively-charged cleft, activated Cas12a2 is able to cleave a variety of nucleic acid substrates. dsDNA is captured in the active site, bent at 90 degrees, melted and held open with ‘aromatic clamp’ residues, then degraded, causing abortive infection. Among single-effector nucleases, this mechanism of abortive infection by degrading dsDNA is completely novel.
Exciting potential applications of Cas12a2
One of the obvious applications of Cas12a2 is in CRISPR diagnostics – it can easily be reprogrammed to detect certain targets, such as RNA viruses, with high specificity. Jackson, Beisel, and their teams have already demonstrated the feasibility of this approach in their recent work.
But that’s not the only possibility for this strange nuclease - Cas12a2 could also be programmed to kill specific cell types, such as tumour cells, for therapeutic applications. Jackson and Beisel have only scratched the surface of the capabilities and potential applications of Cas12a2 and its relatives in their recent papers, but work is ongoing.
»There's a lot of possibilities in terms of the applications of Cas12a2. At the moment we’re just figuring out what it does in different contexts, because this nuclease is full of surprises. I’m most excited to see what it will do in human cells – that's something we're actively investigating. There also seems to be a broad diversity of these Cas12a2 nucleases that we're just starting to learn about, and we think that they have other capabilities besides what we reported in these papers. « Beisel concludes.
Links to the original articles in Nature:
Cas12a2 elicits abortive infection through RNA-triggered destruction of dsDNA
RNA targeting unleashes indiscriminate nuclease activity of CRISPR–Cas12a2
Rebecca Roberts is a molecular biologist and science writer/communicator based in Queensland, Australia.
To get more of the CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
CLINICAL TRIALS
Sponsors:
Suzhou Maximum Bio-tech Co., Ltd.
Sponsors:
Zhejiang University







