Clinical Trial Update: Caribou’s CB-010 Rivals Response Rates and Safety Profiles of Approved Autologous CAR-T Cell Therapies
Last week, Caribou Biosciences reported positive long-term follow-up data from the dose escalation portion of the ongoing ANTLER Phase 1 trial. The trial, which commenced in May 2021, is evaluating Caribou's lead allogeneic CAR-T cell therapeutic candidate CB-010 for the treatment of relapsed or refractory B cell non-hodgkin lymphoma (r/r B-NHL).
The new data set includes all 16 patients with aggressive r/r B-NHL who were treated with one of three dosing levels of CB010 (40x106, 80x106, and 120x106 CAR-T cells). Each of these 16 patients had either received two or more prior lines of chemoimmunotherapy or were primary refractory patients (i.e. never achieved a complete remission from any therapy).
In its press release, Caribou Biosciences states that data for CB-010 rivals response rates and safety profiles of approved autologous CAR-T cell therapies, with an overall response rate of 94 % and a complete response rate of 69 % after a single dose of CB-010.
Overall response rate and complete response rate
According to the U.S. National Cancer Institute (NIH), the overall response rate (ORR) is the percentage of individuals in a study or treatment group that exhibit a partial (PR) or complete response (CR) to the treatment within a certain period of time. CR specifically refers to the disappearance of all signs of cancer in response to treatment (also known as remission). Source: National Cancer Institute.
First PD-1 knockout CAR-T candidate to enter clinical trial
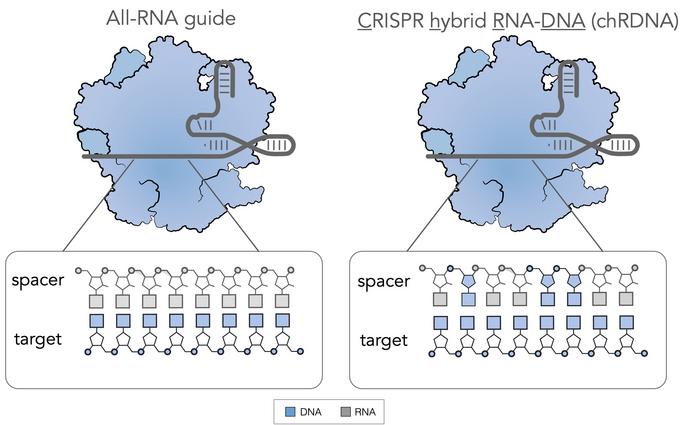
CB-010 is designed to be an 'off' the shelf' therapy, which is derived from healthy donor T cells that are edited using Caribou's proprietary Cas9 chRDNA technology.
Unlike conventional CRISPR-Cas gene editing, which involves the use of all-RNA guides, Caribou uses CRISPR hybrid RNA-DNA guides (chRDNAs), which are generated by carefully introducing DNA into the backbone of an RNA guide (Figure 1).
chRDNA technology was developed following discoveries by Caribou scientists that strategically introducing DNA into an RNA guide led to a detuning of the affinity when there's a mismatch between the guide RNA and a region of genomic DNA. You can read more about Cas9 chRDNA in our interview with Caribou's CSO Steve Kanner Ph.D. here.
To generate CB-010, a CD19-specific CAR is inserted into the TRAC gene (which encodes the T cell receptor alpha constant) of healthy donor T cells. The PD-1 gene is also deleted in these cells. This gene encodes the PD-1 protein that functions as a safety switch on T cells that cancer cells turn on to protect themselves from T cell-mediated immune responses. In 2020, CB-010 became the first allogeneic CAR-T cell therapy with a CRISPR-mediated PD-1 deletion to receive FDA clearance for clinical trial initiation.
CB-010 treatment is safe and effective after a single dose
The new data released last week revealed that CB-010 was generally well tolerated across all three dosing levels, with any adverse events observed consistent with autologous or allogeneic CD19-targeting CAR-T cell therapies. The company reported no dose-limiting toxicities (DLT) at dose levels 2 or 3 following a single DLT at dose level 1.
Specifically for patients with r/r B-NHL, Caribou Biosciences reported a 94% overall response rate (ORR) following a single dose of CB-010, which corresponds to a therapeutic reponse to CB-010 in 15 of 16 patients. 11 out of 16 (or 69 % of) patients were reported to acheive a complete response (CR) to CB-010. Seven of 16 patients (44%) had a CR at ≥6 months following a single dose of CB-010, while 24 months is the longest CR maintained to date.
For a subset of 10 patients with large B cell lymphoma (LBCL) treated with CB-010, a 90% ORR (9 of 10) was observed. 70% (7 of 10) of this subgroup achieved a CR, while 50% (5 of 10) had a CR at ≥6 months, with18 months being the longest CR maintained to date.
Dose expansion continues in ANTLER trial, initial data expected in 2024
Based on these data, Caribou is enroling second-line patients with LBCL in the ongoing dose expansion portion of the ANTLER clinical trial. Here, the mid- and the high doses from escalation (80x106 and 120x106 CAR-T cells) are being evaluated in approximately 15 patients per dose level to determine the recommended Phase 2 dose. Once this is determined, the company may enrol additional patients in ANTLER.
Caribou plans to report initial dose expansion data from the ongoing ANTLER trial in H1 2024.
For a complete overview of CRISPR IND approvals and ongoing gene-editing clinical trials, check out CRISPR Medicine News' Clinical Trials Database.
To get more CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
ArticleNewsClinical News UpdatesNon-Hodgkin Lymphoma, NHLCaribou Biosciences, Inc.
CLINICAL TRIALS
Sponsors:
Suzhou Maximum Bio-tech Co., Ltd.
Sponsors:
Zhejiang University







