Clinical Trial Update: Positive Clinical Data for First Ever CRISPR Therapy for HIV
San Francisco-headquartered Excision BioTherapeutics shared positive interim clinical data on its CRISPR-based therapeutic candidate for HIV at last week’s European Society for Gene & Cell Therapy annual meeting.
The company is developing EBT-101 as a potential functional cure for chronic HIV infection by targeting latently integrated proviral RNA. The ongoing Phase 1/2 trial is evaluating the safety and pharmacodynamics of EBT-101 in individuals living with HIV type 1 (HIV-1), which is the subtype present in about 95 % of HIV cases worldwide.
The data from the first dose cohort included positive safety and biodistribution data to 48 weeks. The company reported no serious adverse events or dose-limiting toxicities in any of the first three trial participants dosed with EBT101, and that EBT-101 could be detected in the bloodstreams of those three trial participants.
Based on these data, the company is planning to initate testing in the next dose cohort in Q4 2023 and it expects to present further clinical data in 2024.
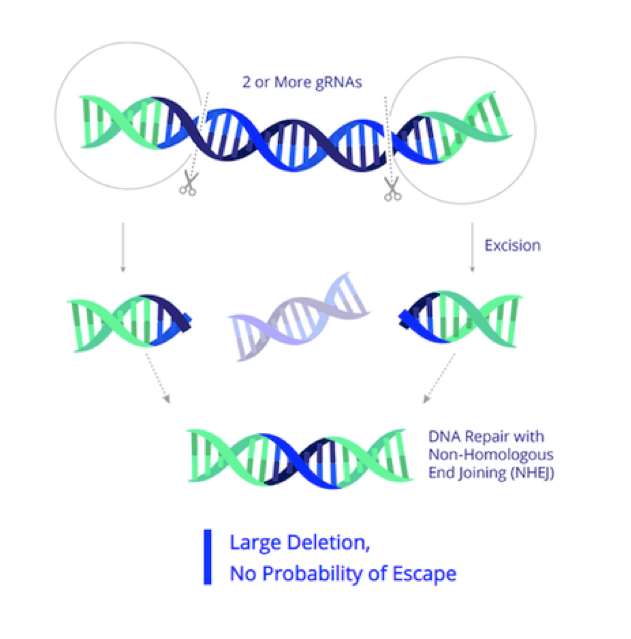
EBT-101 is developed as an in vivo CRISPR therapy
HIV is an RNA virus, and when it first infects an individual, its RNA is reverse-transcribed into DNA by virally-encoded reverse transcriptase and RNase H enzymes that convert the viral RNA to double-stranded DNA (dsDNA) and degrade any remaining viral RNA, respectively. The resulting dsDNA is integrated into the host genome and this is referred to as proviral RNA. Proviral RNA is a serious barrier to curing HIV, since this is not eliminated by the currently available antiretroviral therapies.
EBT-101 is designed to excise HIV proviral DNA using CRISPR-Cas9 and two guide RNAs (gRNAs). The CRISPR cargo is delivered to cells as a one-time treatment via an adeno-associated virus (AAV). The dual gRNAs target three sites within the HIV genome, thereby cutting out large portions of the HIV genome and minimising potential viral escape.
In 2021, Excision Biotherapeutics announced that the FDA had cleared its HIV candidate EBT-101 for clinical trial initiation in the US. The company announced in July 2023 that EBT-101 had been granted Fast Track Designation by the FDA.
To date, EBT-101 is the only CRISPR-based therapy for HIV to receive IND clearance by the FDA.
Pre-clinical studies demonstrate curative potential
Previously reported pre-clinical studies carried out in the lab of Kamel Khalili at Temple University in Philadelphia, which is associated with Excision BioTherapeutics, demonstrated that EBT-101 could remove HIV proviral DNA from multiple cell lines as well as the genomes of transgenic mice. This was the first demonstration of a functional cure for HIV in animals, and the work was carried out in collaboration with Dr. Howard Gendelman's group at the University of Nebraska Medical Center.
Subsequent proof-of-concept studies shed similar findings in rhesus macaque monkeys infected with the related simian immunodeficiency virus (SIV), where a single systemic treatment with AAV9-delivered Cas9 and dual gRNAs could remove proviral DNA from the genomes of blood cells and other tissues known to be viral reservoirs.
EBT-101 is Excision’s lead programme, and the company’s proprietary viral excision technology was developed at Kamel Khalili's lab at Temple University and Jennifer Doudna’s lab at UC Berkeley.
EBT-101 clinical trial (EBT-101-001)
This is a First in Human (FIH), open-label, sequential cohort, single ascending dose (SAD) study of EBT-101, which will be administered intavenously (IV) to approximately nine aviremic HIV-1 infected adults on stable antiretroviral therapy (ART).
On Day 1, eligible participants will receive a single IV dose of EBT-101. All participants will be assessed for eligibility for an analytical treatment interruption (ATI) of their background ART at Week 12. All participants will be followed through Week 48, and participants who undergo ATI will have more frequent study visits than non-ATI participants.
Eligible participants who are enrolled in this study will also be enrolled in a separate long-term follow-up (LTFU) study (EBT-101-002) for safety monitoring. The duration of the LTFU study will be up to 15 years.
To get more CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
ArticleNewsClinical News Updatesin vivoAdeno-associated virus (AAV)Human Immunodeficiency Virus Infection, HIVInfectious diseaseCRISPR-CasExcision BioTherapeutics
CLINICAL TRIALS
Sponsors:
Suzhou Maximum Bio-tech Co., Ltd.
Sponsors:
Zhejiang University







