CMN Weekly (1 November 2024) - Your Weekly CRISPR Medicine News
By: Gorm Palmgren - Nov. 1, 2024
Top picks
- A single-cell CRISPR-based molecular clock was developed to retrospectively time cellular events by encoding genetic marks in vivo. Applied to mouse embryos, it revealed precise cell expansion timing, unconventional lineage relationships, and new epithelial progenitor states. In mouse and human precancer studies, polyclonal initiation in 15–30% of colonic precancers was identified, establishing a multimodal framework for understanding development and tumour origins.
- MetaCRISPR typing using low-biomass skin samples might help with personal identification in forensics, even when human DNA analysis fails. Sequencing of CRISPR spacers in the microbiome required a minimum of 102 copies for reproducibility. In six human DNA-deficient samples, three yielded reliable identification indices via metaCRISPR typing, suggesting its forensic value where traditional DNA methods fall short.
Research
- A novel electroporation device, the LaViE-Chip, improves CRISPR-Cas9 therapeutic potential by enabling continuous, large-scale gene editing. In HEK293T cells, its parallel microfluidic channels and optimised curvature achieve 71% transfection efficiency and 84.3% cell viability, with a rapid processing rate of 107 cells/min.
- A new study investigates how CRISPR-Cas9 identifies DNA mismatches by stabilising a "surveillance state" that enables high-fidelity DNA proofreading. Utilising NMR techniques, researchers identified a conformational switch in the REC3 domain of Cas9 that modulates DNA cleavage by the HNH domain, enhancing on-target specificity and providing insights into developing more accurate CRISPR applications.
- CRISPR-Cas9 was used to correct a specific sixth exon mutation in the CASR gene in patient-derived hiPSCs linked to neonatal severe primary hyperparathyroidism, leaving a single heterozygous mutation in the seventh exon. The edited hiPSC line showed normal karyotype, pluripotency, and differentiation potential, providing a robust model for exploring Ca-homeostasis and personalised drug applications for related disorders.
- Researchers in Iran and Germany have developed a deep-learning model, DTMP-Prime, to improve predictions for Prime Editing (PE) efficiency and pegRNA activity. The tool can be used to select the best pegRNA and nick gRNA (ngRNA) combinations and accurately predict the efficiency and outcomes of PE experiments.
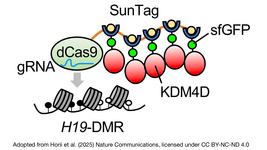
- A new frontier in prenatal gene editing emerges after successful in vivo delivery of mRNA via acid-degradable nanoparticles into the brains of fetal mice. The new method uses densely PEGylated lipid nanoparticles to deliver CRISPR-based mRNA editing tools into embryonic brain cells, achieving substantial genetic modifications across the brain with minimal toxicity and long-lasting results.
- BES-Designer is a new web tool that facilitates guide RNA (gRNA) design for CRISPR-Cas base editing by simplifying target sequence selection for diverse PAMs and editing types. Using rule-based sequence simplification, BES-Designer achieves a 30% efficiency gain in base-editing library creation.
- A new compact RNA-targeting nuclease, Cas13bt3, is demonstrated to be suitable for AAV delivery as an effective anti-VEGF agent for retinal therapies. Expression of Cas13bt3 and a single guide RNA (sgRNA) targeting exon 4 in the VEGFA gene in retinal cells reduced VEGFA mRNA levels significantly in both human retinal organoids and VEGF-overexpressing transgenic mice.
Industry
- Allogene Therapeutics has announced that its CAR T candidate ALLO-316 - based on TALEN (Transcription Activator-Like Effector Nuclease) gene-editing technology - recently received the FDA's Regenerative Medicine Advanced Therapy (RMAT) designation for treating CD70-positive advanced or metastatic renal cell carcinoma (RCC). This designation aims to speed up the development and regulatory review of products that show potential to address unmet medical needs in serious conditions.
- At the recent ESGCT Congress, Precision BioSciences has demonstrated ARCUS nuclease efficiency in gene insertion, achieving over 85% homology-directed repair (HDR) integration in T cells and hepatocytes using AAV6 vectors. This targeted insertion, enabled by ARCUS' staggered 3' overhang cut, facilitates base edits, deletions, and large genomic replacements.
Perspectives
- The Global Legal Post brings the latest twists in the CRISPR patent saga. The CVC group, representing Nobel winners Charpentier and Doudna, recently withdrew two key European CRISPR patents ahead of an appeal, citing procedural concerns. This revocation, criticised as tactical, avoids a potentially adverse ruling while highlighting shifts in European Patent Office practices. The Board of Appeal's stance may prompt patentees to file auxiliary claims earlier, potentially impacting CRISPR-related IP strategy and revocation cost responsibilities.
Reviews
- Leveraging CRISPR gene editing technology to optimise the efficacy, safety and accessibility of CAR T-cell therapy. This review explores how CRISPR-Cas technology optimises CAR T-cell therapy by enhancing its effectiveness, safety, and accessibility through precise gene editing and high-throughput screening techniques.
- Engineered exosomes in service of tumor immunotherapy: From optimising tumor-derived exosomes to delivering CRISPR/Cas9 system. This review explores different techniques for enhancing TEXs using various engineering strategies and discusses how these exosomes can be incorporated into advanced genetic engineering systems like CRISPR/Cas9 for possible therapeutic uses.
- Unlocking Genome Editing: Advances and Obstacles in CRISPR/Cas Delivery Technologies. This review attempts to provide a comprehensive survey of the existing CRISPR-Cas9 delivery strategies, including viral delivery, biomaterials-based delivery, synthetic carriers, and physical delivery techniques.
News from CRISPR in agriculture
- Tuesday we published a newsletter from our sister site, CRISPR AgroBio News (CARBON) that brings you the latest news on how CRISPR can shape agriculture for the future to guarantee food security in times of population growth and climate change. Check it out!
To get more CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
ArticleMissing linksNewsCMN WeeklyAllogene Therapeutics, Inc.Precision BioSciences, Inc.
CLINICAL TRIALS
IND Enabling
Phase I
Phase II
Phase III
Gastric Cancer and Colorectal Cancer, CRC, (NCT07166263)
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
IND Enabling
Phase I
Phase II
Phase III
Relapsed or Refractory Acute Myeloid Leukemia, AML, (NCT06541444)
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
IND Enabling
Phase I
Phase II
Phase III