CMN Weekly (8 December 2023) - Your Weekly CRISPR Medicine News
By: Gorm Palmgren - Dec. 8, 2023
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...
Top picks
- Researchers at Precision BioSciences and elsewhere have presented the development of mitoARCUS, a mitochondrial-targeted nuclease that offers a promising approach for editing heteroplasmic mitochondrial DNA (mtDNA). Based on the small, highly specific ARCUS nucleases derived from I-CreI, this technology is designed to target and eliminate mutant mtDNA, specifically the common m.3243A>G mutation. It successfully removes mutant mtDNA without affecting wild-type DNA, improving mitochondrial function. As demonstrated in a xenograft mouse model using adeno-associated virus delivery, mitoARCUS shows potential as an in vivo gene-editing therapy for diseases associated with this mutation.
- American researchers have successfully used adenosine base editors, optimised through testing over 100 guide RNAs and base editors, to target the SMN2 exon-7 mutation in spinal muscular atrophy (SMA). This approach, leveraging high-fidelity Cas9 variants, reverted the exon-7 mutation in up to 99% of patient-derived fibroblasts, significantly increasing SMN2 exon-7 transcript and SMN protein levels. This CRISPR-Cas-based method offers the potential for durable SMA treatments.
Research
- Utilising CRISPR screening, the deubiquitinase ATXN3 has been identified as a key regulator of PD-L1 transcription in tumour cells, a critical factor in tumour immune evasion. The screen found ATXN3 to positively influence PD-L1 transcription, aiding tumour cells in evading the immune system.
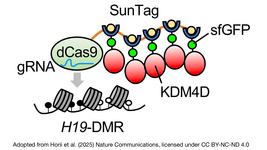
- Researchers in South Korea present a novel CRISPR-Cas9 strategy to combat hepatitis C virus (HCV) recurrence post-liver transplantation. An RNA aptamer targeting HCV's NS5B replicase was integrated into the AAVS1 "safe harbour" site in a hepatocellular carcinoma cell line. The genome editing resulted in stable aptamer RNA expression, effectively inhibiting HCV at RNA and protein levels and conferring HCV resistance.
- Chinese researchers have developed DNA origami nanocages that release CRISPR-Cas9 only in the presence of specific tumour markers like ATP or miRNA-21. This method ensures targeted gene editing directly within tumour cells, significantly reducing their growth. This innovative approach offers a precise and effective way to apply CRISPR to treat tumours, demonstrating success in laboratory and live models.
- Using CRISPR-Cas9, researchers corrected a key COL7A1 gene mutation in 58% of cells from Recessive Dystrophic Epidermolysis Bullosa (RDEB) patients. Grafting these corrected cells onto mice led to improved skin protein formation and structure in mouse models, showing potential for effective gene therapy in RDEB treatment.
- Chinese researchers successfully used CRISPR-Cas9 to target the vanA gene in vancomycin-resistant bacteria, reducing antibiotic resistance. By designing specific sgRNAs to cleave vanA, the research showed a marked decrease in vanA-harboring plasmids and vancomycin resistance, highlighting CRISPR-Cas9's potential in combating antibiotic resistance.
- An Australian study has utilised CRISPR gene editing on iPSCs to classify a GATA4 genetic variant in congenital heart disease. Efficient CRISPR editing and cardiomyocyte modelling compared variant and healthy cells, revealing changes in calcium and adrenergic signalling and altered action potentials consistent with patient symptoms. This method effectively interprets genetic variants in cardiac diseases, offering quicker, in vivo variant assessment and potential applications to other genes.
- Greek researchers present a novel strategy for the detection of CRISPR-induced indels. The method relies on PCR-induced mutagenesis-restriction fragment length polymorphism (PIM-RFLP). It allows for assessing the editing efficiency in pools of edited cells and the effective identification of fully mutated single-cell clones.
- In a study on B-cell acute lymphoblastic leukaemia (B-ALL), CRISPR-Cas9 loss-of-function screens revealed IFNγR/JAK/STAT signalling and antigen processing as key resistance mechanisms to CAR-T therapy. Surprisingly, these pathways were upregulated in relapsed tumours and B-ALL patients with poor CAR-T outcomes. The study highlights a novel resistance mechanism involving natural killer cells and tumour-cell interaction within the in vivo microenvironment.
- The S315 peptide, improving upon the S10 shuttle peptide, effectively delivers base editor RNP for gene editing in airway epithelial cells. In rhesus macaques, intratracheal aerosol delivery of S315 showed widespread respiratory tract distribution. Editing efficiencies reached up to 5.3% in rhesus airway epithelia, with edited cells persisting for 12 months in mice. Crucially, ABE8e-Cas9 targeting the CFTR R553X mutation in human cells restored anion channel function, underscoring S315's potential in treating cystic fibrosis.
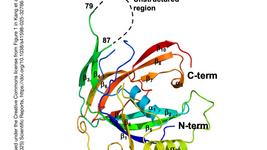
- A new compact CRISPR-Cas9 system, EvCas9, from Eubacterium ventriosum, offers broader therapeutic applications due to its smaller size (1107 residues) and unique PAM specificity (5′-NNGDGN-3′). Its mechanism, revealed through cryo-EM structural analysis, shows efficient genome editing in bacteria and human cells with higher fidelity than SaCas9 and SpCas9. EvCas9 has been further engineered for base editing, expanding the CRISPR-Cas9 toolbox and enhancing our understanding of its working mechanisms.
Industry
- Days before the expected FDA approval of its sickle cell gene therapy, Casgevy, CRISPR Therapeutics unveils revisions to its immuno-oncology portfolio by focusing on second-generation CAR T cell candidates CTX112 and CTX131. Most significantly, the company is also expanding its trials into autoimmune diseases, particularly with CTX112, which is showing great promise in this regard.
- Eligo Bioscience has announced a successful $30 million Series B funding round. The funding is expected to generate proof of concept clinical data in patients and propel Eligo towards becoming a clinical-stage biotech.
- Kamau Therapeutics has announced its emergence from stealth following a strategic transaction with Graphite Bio. Under the terms of the agreement, Kamau receives an option to acquire all genome editing assets, including a platform technology that integrates precision DNA repair using homology-directed repair (HDR) and CRISPR-Cas9.
Detection
- Chinese researchers have introduced a LAMP-CRISPR-Cas12b assay for quick and precise detection of Chlamydia psittaci. The method detects the pathogen in under an hour, showing no cross-reactivity and matching qPCR accuracy in clinical tests. With detection limits of 102-103 aM, it offers a rapid diagnostic tool for psittacosis (parrot fever).
Reviews
- Engineered deaminases as a key component of DNA and RNA editing tools. This review summarises the current trends and achievements in deaminase engineering and presents data indicating that the potential of these enzymes has yet to be fully revealed or understood.
- Global research trends in CRISPR-related technologies associated with extracellular vesicles from 2015 to 2022: a bibliometric, dynamic, and visualised study. This review explores the emerging trends, dynamic development, and research hotspots of CRISPR technology associated with extracellular vesicles during the past seven years and demonstrates them by visualisation.
- DNA on the move: mechanisms, functions and applications of transposable elements. This review provides a broad overview of the molecular mechanisms underpinning DNA transposition - including bacterial CRISPR-associated transposons (CASTs) - and its biological and technological impact.
- Drug addiction and treatment: An epigenetic perspective. This review discusses how epigenetic editing has been used in various neuropsychiatric conditions and how initial results in addiction treatment involving model organisms have fallen out.
- Potential therapeutic strategies for osteoarthritis via CRISPR/Cas9 mediated gene editing. This review aims to epitomise the role of CRISPR as an analogous therapeutic strategy that could greatly facilitate the clinical development of osteoarthritis-related treatments for osteoarthritic patients.
- Is It Ever Wise to Edit Wild-Type Alleles? Engineered CRISPR Alleles Versus Millions of Years of Human Evolution. This review evaluates the CRISPR-Cas strategy of knocking out PCSK9 to reduce plasma LDL and cardiovascular disease in the light of PCSK9's other roles in, e.g., infection and immunity.
Perspectives
- While we await the FDA's first approval of a CRISPR therapy - Casgevy for sickle cell disease - a perspective in Nature looks at the next generation of CRISPR techniques. The piece emphasises that while basic CRISPR-Cas9 therapies can do some remarkable things, they represent the first generation of genome editing. Next-generation systems like base editing, prime editing and epigenome editing edit DNA with more precision and versatility and will lead to more effective and safe therapies.
News from CRISPR Medicine News
- On Monday, we posted an explainer about prime editing. Prime editing is considered to have a desirable safety profile compared to conventional CRISPR-Cas9-mediated gene editing approaches because it allows for targeted DNA editing without intentionally generating DNA double-strand breaks.
To get more of the CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
CLINICAL TRIALS
IND Enabling
Phase I
Phase II
Phase III
Gastric Cancer and Colorectal Cancer, CRC, (NCT07166263)
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
IND Enabling
Phase I
Phase II
Phase III
Relapsed or Refractory Acute Myeloid Leukemia, AML, (NCT06541444)
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
IND Enabling
Phase I
Phase II
Phase III