CRISPR Ameliorates Disc Degeneration and Reduces Pain Signals in Mouse Model of Back Disc Injury

Despite numerous efforts to reverse intervertebral disc degeneration and alleviate lower back pain using cell therapy, biomaterial-based disc repair, and gene therapy, surgery remains the mainstay in the treatment of disc degeneration.
In a recent study, researchers from the Department of Orthopedic Surgery, Rush University Medical Center (Chicago, USA) developed a new CRISPR-Cas9-based method for treating disc degeneration by targeting β-catenin, a key signalling molecule that has been implicated in disc degeneration.
»In a proof-of-concept study, we showed that targeting β-catenin using CRISPR-Cas9 significantly mitigated multiple pathological changes of disc degeneration in a mouse model of disc injury,« said Lan Zhao, PhD, Assistant Professor at Rush Medical College and co-first author of the study.
Dr Zhao and her co-workers found that targeting β-catenin ameliorated various pathomorphological alterations associated with disc degeneration. In addition to restoring the structure of the disc matrix, silencing β-catenin also attenuated pain-related neural events in a mouse model of disc degeneration.
»Our data suggest that using CRISPR-Cas9 to target β-catenin in disc cells may help ameliorate disc degeneration and back pain,« Dr Zhao noted. »Although we do not expect that targeting β-catenin using viral vectors would be readily available for the clinical management of disc degeneration, we anticipate that a compound or biologic antagonising β-catenin or the Wnt signalling pathway could be useful,« she added.
The team’s findings were published in Molecular Therapy Nucleic Acids.
The critical role of β-catenin in disc degeneration
Disc degeneration, a major cause of back pain-related disability, is a complex process characterised by marked changes in the disc structure. Many of the biological pathways associated with disc degeneration are regulated by cytokines and growth factors, and these pathways are at the forefront of efforts to identify therapeutic targets for disc degeneration. However, there are not many in vivo studies of gene function in animal models of disc degeneration, limiting our understanding of the molecular changes that promote disc degeneration.
In vitro and in vivo evidence suggests that Wnt/β-catenin signalling is a key regulator of disc development and disc metabolism. Additionally, samples from patients with intervertebral disc degeneration show elevated levels of β-catenin, which is encoded by the Ctnnb1 gene, providing clinical clues to the pivotal role of Wnt/β-catenin signalling in disc degeneration.
»β-catenin is a master transcription factor in skeletal biology, and activation of β-catenin has been shown to drive disc degeneration. Therefore, we hypothesised that using CRISPR-Cas9 to ablate β-catenin expression may be a promising strategy for attenuating disc degeneration,« said Jian Huang, PhD, Assistant Professor at Rush Medical College and corresponding author of the study.
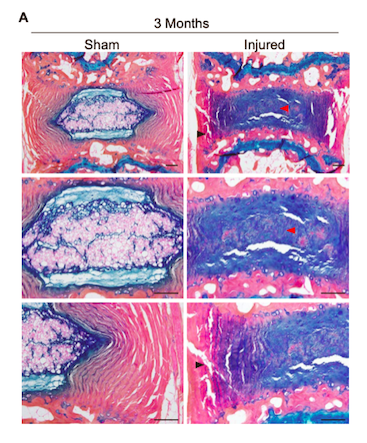
Creating a mouse model of disc injury
The team first established a mouse model of disc injury by inserting a needle into the tail disc of mice, thereby injuring the tail disc. Disc tissues in mice three months after stab injury showed degenerative changes, with profound decompression of the nucleus pulposus, loss of notochordal cells, and disorganisation of the annulus fibrosus (Figure 1). Some of these changes closely resemble intervertebral disc degeneration in humans.
To confirm β-catenin upregulation in the mouse model of disc degeneration, the team conducted fluorescent immunohistochemistry three and six months after disc injury, after the acute reactions induced by the trauma have resolved. β-catenin was upregulated approximately two-fold in the nucleus pulposus of injured discs, and its expression remained elevated throughout the progression of disc degeneration.
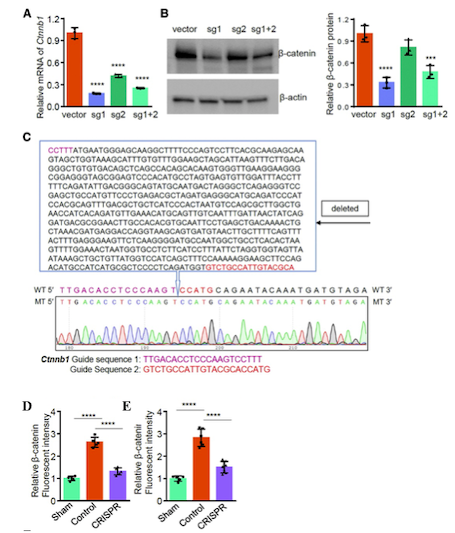
CRISPR-Cas9 treatment reduces β-catenin expression in vitro and in vivo
After inducing injury to the tail discs of mice, the team turned to a CRISPR-Cas9-editing strategy that would specifically target β-catenin.
»For in vivo β-catenin targeting, we chose the CRISPR-Cas9 system derived from Staphylococcus aureus, as SaCas9 is significantly smaller than the majority of Cas9 homologs and, thus, allows efficient AAV packaging,« Dr Zhao explained.
Exposure of mouse mesenchymal cells to AAV vectors expressing SaCas9 and two Ctnnb1-targeting sgRNAs successfully reduced β-catenin expression levels in vitro. Sequencing of the target region in Ctnnb1 showed that CRISPR-Cas9 treatment led to the generation of a frameshift mutation due to a 679-nucleotide deletion in the gene, introducing a premature stop codon that rendered β-catenin non-functional (Figure 2).
To disrupt β-catenin expression in vivo in mouse disc cells, Dr Zhao and colleagues treated mice with AAVs serotype 5 carrying SaCas9 and Ctnnb1-targeting sgRNAs via intradiscal injection. Using this approach, the team observed reduced β-catenin levels by nearly 50% in injured disc tissues, and the reduction in β-catenin levels was maintained for up to six months after treatment (Figure 2).
»A key advantage of using AAV-mediated delivery of CRISPR-Cas9 to target β-catenin is that it enables efficient and long-lasting reduction in β-catenin levels in vivo in disc tissues,« said Dr Huang.
Targeting β-catenin ameliorates disc degeneration and matrix remodelling after disc injury
Targeting β-catenin using CRISPR-Cas9 ameliorated various pathomorphological changes associated with disc degeneration. Immunohistochemical analyses of disc tissue three months after injury and β-catenin disruption revealed that, compared with control mice, CRISPR-edited mice exhibited significant preservation of the nucleus pulposus volume and annulus fibrosus structure.
The ability of CRISPR-mediated β-catenin disruption to preserve notochordal cells, attenuate chondro-osteogenesis in the nucleus pulposus, and maintain the annulus fibrosus structure was confirmed six months after disc injury.
»Although the role of β-catenin as a key transcription factor regulating osteoblast differentiation, osteoclastogenesis, and chondrocyte differentiation is known, little is known about the role of Wnt/β-catenin signalling in notochordal cells,” said Dr Zhao. “Our study revealed the important role of β-catenin in governing the maintenance and differentiation of notochordal cells, as well as the development and homeostasis of intervertebral discs.«
The team showed that depletion of notochordal cells in response to disc injury was accompanied by their replacement with extracellular matrix composed of collagen (types I, II, and X); similar remodelling of the extracellular matrix is observed in human intervertebral disc degeneration. Notably, CRISPR-Cas9-mediated disruption of β-catenin significantly reduced collagen deposition, suggesting that reduction in β-catenin levels attenuated matrix remodelling, fibrocartilage synthesis and calcification after disc injury.
Disc injury also resulted in matrix degradation, which was evident by the significant increase in the levels of the matrix proteases ADAMTS5 and MMP13 in the injured disc. However, targeting β-catenin significantly inhibited matrix catabolism in the early and later phases of disc injury.
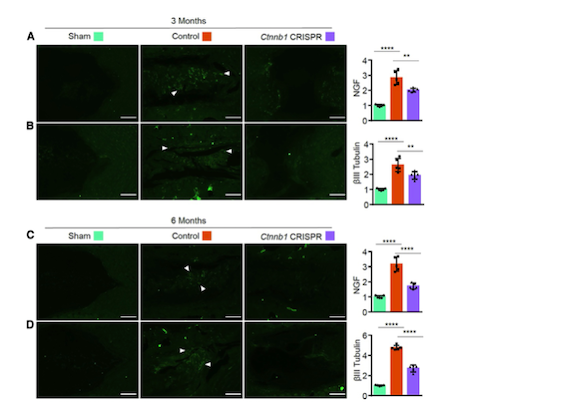
Targeting β-catenin may alleviate pain after disc injury
To assess the potential effects of targeting β-catenin on pain associated with disc degeneration, Dr Zhao and colleagues evaluated the levels of two key regulators of the pain pathway, nerve growth factor (NGF) and βIII tubulin. The discs of control-treated mice had significantly increased levels of NGF and βIII tubulin three and six months after disc injury, suggesting extensive nerve growth and pain sensation (Figure 3). However, CRISPR-mediated β-catenin disruption reduced the levels of NGF and βIII tubulin in the discs of mice three and six months after disc injury.
»Our findings suggest that in addition to ameliorating structural and pathomorphological alterations after disc injury, targeting β-catenin may also suppress pain-related neural growth associated with disc degeneration,« Dr Zhao noted.
Overcoming challenges and next steps
Dr Zhao foresees that the greatest challenge in using CRISPR-Cas9 to treat disc degeneration by targeting β-catenin in humans would be to develop animal models that accurately mimic human disc degeneration.
»In mice, the mature nucleus pulposus is populated by notochordal cells, but in humans, degenerative changes in the nucleus pulposus can be seen as early as two years of age. Degenerative changes in the nucleus pulposus of humans are coincident with the rapid decrease in large vacuolated notochordal cell number following birth,« she explained.
However, despite differences in disc biology between humans and mice, the study demonstrates that notochordal cells are important to disc health. Studies on notochordal cells may provide novel insights and molecular targets for the prophylactic or therapeutic care of disc degeneration.
Dr Zhao also said that, in addition to developing more accurate animal models of disc degeneration, their team is already investigating new therapeutic targets and delivery strategies for the treatment of disc degeneration.
Link to the original article in Molecular Therapy Nucleic Acids:
CRISPR-Cas9-mediated loss of function of β-catenin attenuates intervertebral disc degeneration.
Christos Evangelou, Ph.D., is a freelance medical writer and science communications consultant.
To get more of the CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
CLINICAL TRIALS
Sponsors:
Suzhou Maximum Bio-tech Co., Ltd.
Sponsors:
Zhejiang University







