CRISPR CAR T-Cell Therapy Clinical Update
CTA101 is an off-the-shelf, universal CD19/CD22 dual-targeting CAR T-cell product that is currently being investigated in three Phase 1 clinical trials for relapsed or refractory B cell cancers including B-cell acute lymphoblastic leukaemia (B-ALL) and relapsed or refractory diffuse large B-cell lymphoma (DLB-CL).
The trials are sponsored by Zhejiang University, Xuzhou Medical University, and The First Affiliated Hospital of Nanjing Medical University (all China) in collaboration with various industrial partners in China.
A CRISPR-engineered dual-targeting CAR T-cell product
All three trials aim to assess the safety of CTA101, which is developed using CRISPR-Cas9 technology as a dual-targeting CAR T-cell therapy that targets CD19 and CD22 on the surfaces of cancer cells.
Specifically, CRISPR-Cas9 is used to cut and paste CD19- and CD22-specific CAR coding sequence from a single construct into the T-cell receptor α constant (TRAC) locus in the genome of donor-derived T cells. Since the TRAC locus harbours the T cell receptor (TCR), placing the CAR here abolishes native TCRs while placing the CAR in the regulatory context of the endogenous TCR promoter – a move that has demonstrated improved T cell function and potency in mouse models of acute lymphoblastic leukaemia.
CD52 is also disrupted in the T cells using CRISPR-Cas9. This renders the cells resistant to anti-CD52 antibody therapy, which is often administered prior to CAR T therapy to suppress the patient’s immune system and promote CAR T-cell engraftment.
The dual-targeting CAR approach
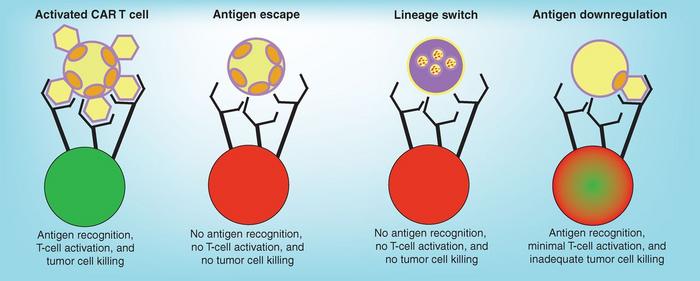
Dual-targeting CARs have been proposed as a potential solution to relapse, which has emerged in a subset of patients as a serious challenge for long-term disease control with CD19-targeting CAR T therapies.
CD19 is a cell surface antigen expressed by all B-cell cancers, but despite initial excellent clinical responses to CD19-targeting therapies, relapse due to antigen escape occurs naturally in some patients, whereby tumour cells lose or down regulate the CD19 antigen.
CD22 is primarily expressed on the surfaces of mature healthy B cells and it is also expressed by most leukaemic cells in the majority of B-cell acute lymphoblastic leukaemias. It has emerged as an attractive target for certain relapsed or refractory B cell cancers, either alone or in combination with CD19 as a dual-targeting therapy.
Phase 1 trial for relapsed or refractory B cell cancers – NCT04227015
The primary objective of this early Phase 1 study is to explore the dose-related safety of CTA101 in a total of 72 child and adult patients with relapsed or refractory CD19+ ALL or Non-hodgkin lymphoma.
The study is single arm, open-label, and single-center, and participants will be divided into two groups of 36. Each group will receive a single injection of either of two doses of CTA101.
Primary outcome measures include a standard assessment of the occurrence of adverse events and the incidence of treatment-emergent adverse events up to 28 days and 2 years post-treatment, respectively. Secondary outcome measures include overall response rates and survival at various intervals post-treatment for the two diseases in question.
This study began in January 2020 and the estimated study completion date is May 2027. Preliminary data shared at the annual American Society of Hematology meeting in December 2020 and published this month in Clinical Cancer Research revealed a manageable safety profile for CTA101 and encouraging signs of efficacy in the 6 patients enrolled at that time, with no occurrence of graft versus host disease or CRISPR-related adverse events.
Phase 1 trial for B-cell acute lymphoblastic leukaemia – NCT04154709
This trial will assess the safety and feasibility of CTA101 to treat patients with relapsed or refractory CD19+ B-ALL. A total of 15 child and adult participants (3 to 70 years of age) will be enrolled in the trial, which is open-label. The participants will receive a single intravenous infusion (by injection) of CTA101 at one of three escalating doses. Dose escalation begins with administration of the lowest dose followed by administration of the ascending doses, but each participant only receives a single infusion. The 3+3 dose escalation design is applied, meaning that the maximum tolerated dose (MTD) is the highest dose at which at most one patient out of six patients (i.e. 17%) experience dose-limiting toxicity (DLT).
Primary and secondary outcome measures include a DLT assessment and overall treatment response rates and survival at various time points following infusion. The trial began in December 2019 and the anticipated completion date is June 2022.
Phase 1 trial for relapsed or refractory diffuse large B-cell lymphoma – NCT04026100
This trial will evaluate the safety and efficacy of CTA101 in a total of 9 adult participants with relapsed or refractory DLB-CL. The study is single-center, open-label and non-randomised and includes dose escalation, where each participant will receive a single injection of either a low, medium, or high dose of CAR T cells.
Primary and secondary outcome measures include an assessment of adverse events and disease control and response rates according to standard assessment criteria at various intervals up to 2 years post-treatment. infusion. Enrollment has not yet begun and the anticipated completion date is December 2022.
For a complete overview of current gene editing clinical trials, check out CRISPR Medicine News' Clinical Trials Database.
Tags
Articlein vivoLentivirus (LV)Acute Lymphoblastic Leukemia, ALLB-cell Malignancy, NHLNon-Hodgkin Lymphoma, NHLCAR-TBioheng Biotech Co., Ltd.Nanjing Bioheng Biotech Co., Ltd.CRISPR-CasCas9The First Affiliated Hospital of Nanjing Medical UniversityXuzhou Medical UniversityTrialsClinical
CLINICAL TRIALS
Sponsors:
Suzhou Maximum Bio-tech Co., Ltd.
Sponsors:
Zhejiang University