CRISPR Clinical Trial Update
This week we focus on two Phase 1/2 trials sponsored by The First Affiliated Hospital of Guangdong Pharmaceutical University (China) in partnership with Guangzhou Anjie Biomedical Technology Co., Ltd. (China) and University of Technology, Sydney (Australia).
The first trial will evaluate a combined vaccine/CRISPR-edited T cell therapy for prostate cancer, and the second will evaluate a CRISPR-edited CAR-T cell therapy for non-small cell lung cancer (NSCLC).
Combined cancer vaccine and CRISPR-edited T cells for prostate cancer
This trial will evaluate the safety and efficacy of a combination therapy for prostate cancer in three patient subgroups (or study arms). The experimental group will receive combination treatment with a prostate cancer vaccine and PD-1 knockout T cells. The other two groups are active comparator groups, where one will receive the vaccine as monotherapy and the other will receive the PD-1 knockout T cells as monotherapy.
The cancer vaccine is custom-prepared ex vivo using patient-isolated peripheral blood mononuclear cells (PBMCs). The cells are isolated and cultured with a recombinant fusion protein PAP-GM-CSF, which consists of prostatic acid phosphatase (PAP) and granulocyte-macrophage colony-stimulating factor (GM-CSF).
PAP is overexpressed in 95 % of prostate cancer tissues and is an attractive target for the development of anti-prostate tumour vaccines within the immunotherapy field. GM-CSF is an immune stimulant that promotes the maturation of PBMCs to dendritic cells, which can then mount an anti-tumour response against cancer cells expressing PAP.
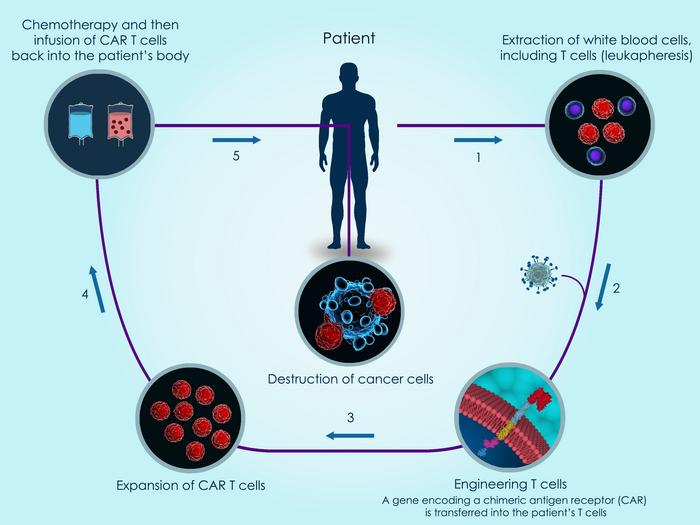
The PD-1 gene encodes the PD-1 protein that functions as a safety switch on T cells that cancer cells exploit to evade T cell-mediated immune responses. PD-1 disruption is sometimes used in CAR T-cell development to enhance anti-tumour activity. The PD-1 knockout engineered T cells are also prepared using patients’ own T cells and the PD-1 gene is knocked out using CRISPR-Cas9 technology.
This trial, which is currently in the recruitment stage, aims to enrol a total of 30 adult males. Each participant will receive either the therapeutic vaccine and/or PD-1 knockout T cells a total of three times over a 2-week period. Primary and secondary outcome measures include an assessment of adverse events and response rate and survival, respectively.
The estimated study completion date is August 2021.
Combined anti-MUC1 CAR-T cells and CRISPR-edited T cells for advanced NSCLC
This study will evaluate the safety and efficacy of anti-MUC1 CAR-T cells as a standalone approach or in combination with PD-1 knockout in patients with advanced NSCLC.
MUC1 (Mucin 1) is a cell membrane glycoprotein that is overexpressed in NSCLC and which has been implicated in carcinogenesis. It is a target in a number of immunotherapeutic approaches to treating NSCLC. Anti-MUC1 CAR-T cells are developed by lentiviral transduction of a MUC1-targeting CAR construct and PD-1 is further knocked out in these CAR-T cells by CRISPR-Cas9 gene editing.
The trial aims to enrol a total of 60 adult participants that will be split into 5 treatment groups where each group will receive either: the CAR-T cells alone, the PD-knockout T cells alone, CAR-T and PD-knockout T cells in combination, an FDA-approved PD-1 monoclonal antibody (mAb), or the patient’s own non-engineered T cells (i.e. a placebo).
Clinical trial data from 20 patients treated in Phase 1 of this study published in 2019 revealed the combined anti-MUC1 PD-1 knockout CAR-T therapy to be well tolerated overall and that multiple treatment cycles are necessary to achieve persistence of the CAR T-cells following infusion. Efficacy of the therapy was inconclusive in that phase of the study.
Secondary outcome measures will evaluate efficacy and these include: response rate, overall survival, progression-free survival and CAR T-cell persistent, each at specific intervals following treatment and in according with up-to-date guidelines for solid tumour assessment.
The anticipated study completion date is January 2022.
For a complete overview of current gene editing clinical trials, check out CRISPR Medicine News' Clinical Trials Database.
Tags
Articlein vivoLung CancerProstate CancerSolid TumoursCAR-TAnjie Biomedical Technology Co., LtdUniversity of Technology, SydneyCRISPR-CasCas9The First Affiliated Hospital of Guangdong Pharmaceutical UniversityTrialsClinical
CLINICAL TRIALS
Sponsors:
Suzhou Maximum Bio-tech Co., Ltd.
Sponsors:
Zhejiang University