ARCUS Gene Editor Shows Promise Against Hepatitis B
The Phase 1/2a ELIMINATE-B trial represents the first clinical evaluation of a gene editor specifically designed to target and eliminate cccDNA. This persistent viral reservoir makes chronic hepatitis B so difficult to cure.
Chronic hepatitis B affects over 250 million people globally. Current nucleoside analogues suppress viral replication but rarely achieve functional cures because they cannot eliminate covalently closed circular DNA (cccDNA) – the stable episomal form persisting in hepatocyte nuclei that serves as the template for viral transcription. Even after years of therapy, cccDNA remains intact, leading to viral rebound when treatment is stopped.
PBGENE-HBV employs Precision's proprietary ARCUS nuclease technology, delivered via lipid nanoparticles containing mRNA. The ARCUS nuclease recognises a highly conserved sequence within the Enhancer 1 element present in both cccDNA and integrated HBV DNA. Unlike other gene editing platforms requiring multiple components, ARCUS functions as a single protein, potentially enabling more efficient access to the compact cccDNA minichromosome (see Figure 1).
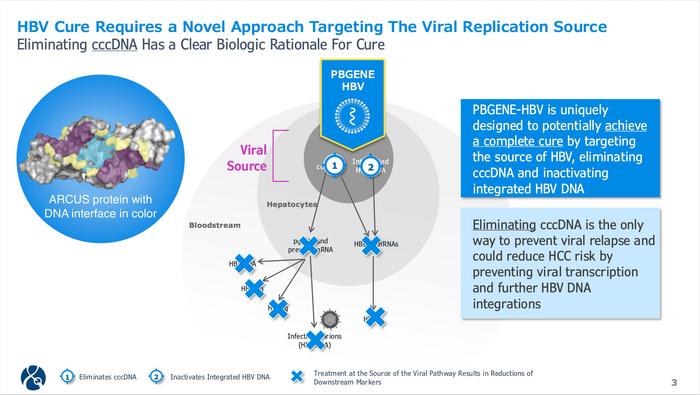
Preclinical studies demonstrated robust efficacy. In non-human primates with AAV-delivered surrogate cccDNA, PBGENE-HBV achieved approximately 50% viral editing after one dose and up to 99% after two administrations. In HBV transgenic mice, treatment produced significant HBV DNA reductions with no viral rebound after nucleoside analogue withdrawal. Safety evaluations revealed no increased risk of chromosomal translocations or germline distribution.
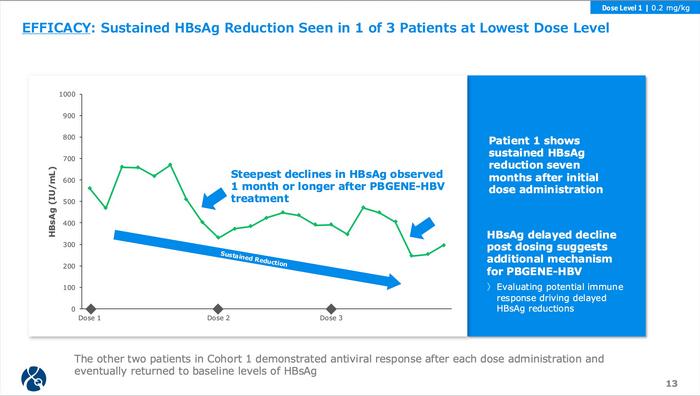
The Phase 1/2a trial enrolled HBeAg-negative patients on stable nucleoside therapy, testing 0.2 mg/kg (Cohort 1) and 0.4 mg/kg (Cohort 2), with up to three infusions eight weeks apart. All three Cohort 1 patients (baseline HBsAg 562-11,813 IU/mL) demonstrated antiviral activity. Maximum HBsAg reductions were 56% (Patient 1), 69% (Patient 2), and 47% (Patient 3). Notably, Patient 1 maintained reduced HBsAg seven months after initial dosing, whilst the others experienced transient declines returning to baseline (see Figure 2).
Safety has been reassuring across both cohorts. Adverse events were mild (Grade 1-2), primarily consisting of transient flu-like symptoms that resolved within hours to days. No serious adverse events or dose-limiting toxicities occurred. Liver enzymes remained well below concerning thresholds – ALT and AST stayed under 150 U/L without sustained elevations approaching the Grade 3 threshold (5x upper limit of normal). Initial Cohort 2 data similarly showed no dose-limiting toxicities through five administrations.
The temporal pattern proved intriguing, with the steepest HBsAg reductions occurring weeks to months post-dosing rather than immediately. This delayed response suggests mechanisms beyond direct genome cleavage, potentially including immune reactivation against infected hepatocytes. The company is investigating whether viral template disruption enables recovery of exhausted HBV-specific T cell responses.
The heterogeneous responses likely reflect the complex biology of chronic HBV. Factors influencing durability may include cccDNA-to-integrated DNA ratios, baseline infected hepatocyte numbers, nuclease delivery efficiency, and individual immune reconstitution capacity. All patients responding at the lowest dose suggest optimisation could enhance both depth and durability of effects.
These results provide proof-of-concept that gene editing can access and disrupt the viral reservoir. PBGENE-HBV represents the first clinical-stage programme directly targeting cccDNA elimination. However, transient responses in two patients highlight challenges in achieving consistent functional cures.
Key questions remain regarding optimal dosing regimens. The company has initiated Cohort 3 and plans to explore shortened dosing intervals and potentially more than three administrations. Understanding which patients will achieve durable responses – potentially through biomarkers like baseline cccDNA levels or immune profiles – will be critical for positioning.
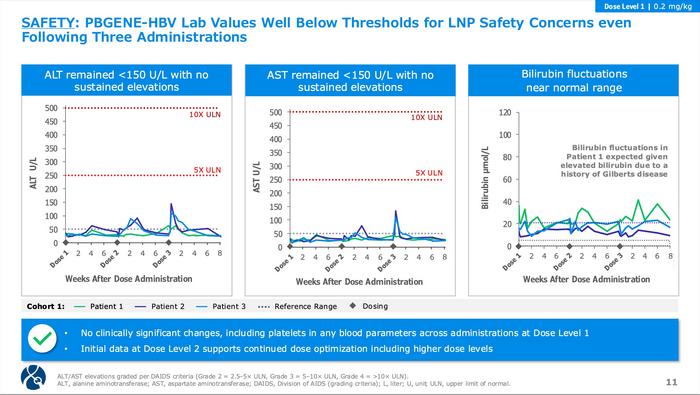
The safety profile supports continued development, though longer follow-up remains essential (see Figure 3). The absence of concerning liver enzyme elevations is particularly encouraging, given the risks of LNP-associated hepatotoxicity. Combination strategies with immune modulators or therapeutic vaccines may prove necessary, especially given the observed delayed HBsAg decline, suggesting an immune contribution to therapeutic effect.
Ongoing enrolment and dose optimisation are planned through 2025 as the company advances this first-in-class approach toward potentially curative therapy for chronic hepatitis B.
The data were presented by Emily B. Harrison, PhD, Director of Translational Science at Precision BioSciences.
To get more CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
ArticleNewsClinical News UpdatesLipid-based nanoparticleHepatitis B virusLiver diseasesARCUSPrecision BioSciences, Inc.Clinical
CLINICAL TRIALS
Sponsors:
Guangzhou Women and Children's Medical Center
Sponsors:
AMERICAN ORGAN TRANSPLANT AND CANCER RESEARCH INSTITUTE LLC