CRISPR Medicine News Company Roundup - CRISPR Therapeutics
As existing technologies advance and new ones emerge, the number of companies working within the CRISPR space is growing rapidly. In this first company-orientated overview from CRISPR Medicine News, we take a look at the clinical pipeline of Swiss biotech company CRISPR Therapeutics, which is one of the key players in the CRISPR medicine field and the first company to show that CRISPR may cure the two major inherited blood diseases sickle cell disease and beta-thalassemia.
CRISPR Therapeutics was founded in 2013, just a year after CRISPR-Cas was first adapted to eukaryotic gene editing. The company was founded by three leading CRISPR researchers; Rodger Novak, Shaun Foy, and Emmanuelle Charpentier who later went on to win the The Nobel Prize in Chemistry 2020 along with Jennifer Doudna of UC Berkeley for outstanding contributions to the CRISPR field.
The Pipeline Frontrunner CTX001
CRISPR Therapeutics' developmental pipeline spans broad disease areas including inherited blood diseases, cancer, diabetes, inherited muscular disorders and cystic fibrosis. There are currently 4 CRISPR-based therapies in clinical development for treatment in 5 disease areas.
The frontrunner is CTX001, an autologous, ex vivo cell therapy that is CRISPR-engineered to reactivate foetal haemoglobin (HbF) in patient-derived haematopoietic stem cells. Upon reinfusion to patients with sickle cell disease or beta-thalassemia, the CRISPR-Cas9-edited cells can produce sufficient levels of HbF to compensate for the adult haemoglobin that is either defective or absent in these diseases.
CTX001 has been in the spotlight recently following encouraging safety and efficacy data from Phase 1/2 studies, and you can read our previous coverage on this programme here.
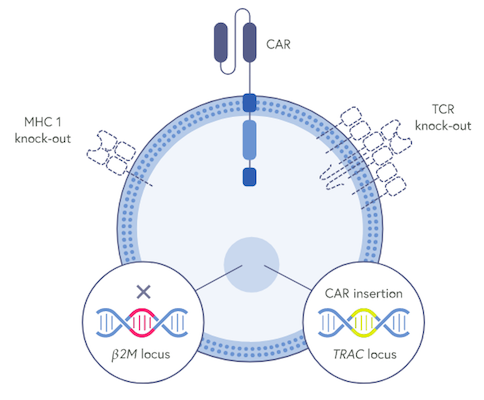
CTX130 - CD70-targeting CAR-T Therapy Candidate
CTX130 is an allogeneic (donor-derived) CAR-T cell therapy that is designed to target the cell-surface antigen CD70, which is expressed by several haematological and solid cancers. The new therapy is developed using T cells from healthy donors that undergo several ex vivo CRISPR-Cas9 modifications to make the product suitable for off-the-shelf CAR-T cell therapy.
Firstly, CRISPR-Cas9 is used to engineer the chimeric antigen receptor (CAR) that allows the CTX130 cells to target and kill CD70-expressing cells. A CAR has two key domains; one that binds to CD70 in this case and another that activates the T cell.
CRISPR-Cas9 is also used to insert the anti-CD70 CAR construct precisely into the T cell receptor (TCR) alpha constant (TRAC) locus. This clever move disrupts the native TCR, thus mitigating the risk of graft-vs-host disease (GVHD) that may otherwise result from histo-incompatibility between a donor and recipient. This edit also places the CAR under endogenous TCR regulation.
CRISPR-Cas9 is further used to eliminate the class I major histocompatibility complex (MHC I) expressed on the surface of the CTX130 cells. This edit alleviates the risk of the patient rejecting the CAR-T product via MHC I, thus increasing the likelihood of the therapy persisting in the patient’s blood.
CRISPR Therapeutics is currently sponsoring 2 clinical trials for CTX130. One trial will assess CTX130 for the treatment of relapsed or refractory T or B cell cancers including certain lymphomas and the other trial will assess safety and efficacy in renal cell carcinoma, a solid cancer of the kidney.
Both trials are single-arm, open-label, multicenter Phase 1 studies evaluating safety and efficacy of CTX130, and the company in Q4 of last year that the first patients had begun treatment with CTX130.
CTX120 - A Novel T Cell Immunotherapy for Multiple Myeloma
CTX120 is an allogeneic T cell therapy that is engineered using CRISPR-Cas9 to target the B-cell maturation antigen (BCMA), which is expressed in multiple myeloma (MM) and which is emerging as a very promising target for this disease.
CTX120 is developed similarly to CTX130, using CRISPR-Cas9 gene-editing to disrupt the native TCR locus and MHC class I, as well as to specifically insert the CRISPR-engineered BCMA-targeting CAR at the TRAC locus.
CTX120 is being investigated in an on-going Phase 1 single-arm, open-label multi-center trial to evaluate its safety and efficacy for the treatment of relapsed or refractory MM over several doses.
Based on intermittant progress in the programme, CRISPR Therapeutics announced last July that CTX120 had been granted Orphan Drug designation from the FDA. The CTX120 trial is anticipated to enroll up to 88 patients in the US, Australia and Canada, and according to a company update in October 2020, enrolment was still going. In that same update, the company also shared that it expects to report initial data for CTX120 this year.
CTX110 - A CD19-Targeting T Cell Immunotherapy
CTX110 is a healthy donor-derived gene-edited allogeneic CAR-T investigative therapy designed to target CD19, a B cell-specific cell surface antigen expressed in all B cell lineage malignancies. CTX110 is developed ex vivo using CRISR-Cas9 technology in a one-step multiplex gene-editing workflow. These edits target a CAR to CD19, disrupt MHC I to improve persistance, and ensure insertion of the recombinant CAR into the TRAC locus.
CTX110 is being investigated in two clinical trials for cancer. The first is CARBON, a Phase 1 open-label, multicenter study to evaluate the safety and efficacy of several doses of CTX110 in adult patients with relapsed or refractory non-Hodgkin lymphoma, who have received at least two prior lines of therapy. The second trial, is a single-arm, open-label, multicenter study to assess safety and efficacy of CTX110 in patients with relapsed or refractory B cell cancer.
In October 2020, CRISPR Therapeutics reported positive top-line data from the CARBON trial of CTX110 in patients with relapsed or refractory B-cell malignancies, with signs of dose-dependent efficacy, acceptable safety and comparable response rates to the early autologous CAR-T trials
Tags
ArticleBlood diseaseCancerCAR-TCRISPR-CasCRISPR Therapeutics AGTrialsClinical
CLINICAL TRIALS
Sponsors:
Suzhou Maximum Bio-tech Co., Ltd.
Sponsors:
Zhejiang University







