Fate Therapeutics Initiates Phase 1 Trial in HER2-Positive Advanced Solid Tumours
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...
Fate Therapeutics announced this week that it has initiated patient enrolment for a Phase 1 clinical trial of FT825/ONO-8250 in solid tumours that express the human epidermal growth factor receptor 2 (HER2). HER2 is a receptor tyrosine kinase that is overexpressed by many solid tumours, including breast, gastric, bladder, and lung cancers.
The Phase 1 trial is being conducted under a strategic collaboration with Ono Pharmaceutical.
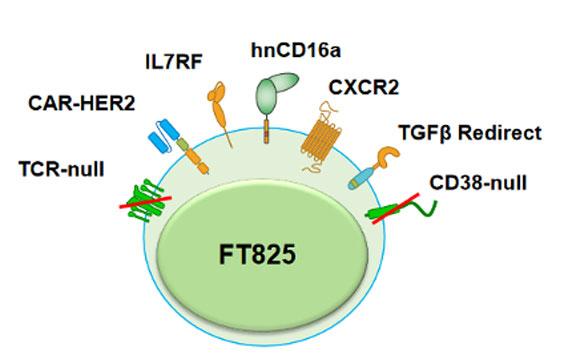
FT825 is a multiplex-CRISPR-edited CAR T-cell candidate derived from induced pluripotent stem cells (iPSCs). The candidate, which is developed using Fate’s proprietary iPSC platform, incorporates a novel HER2-targeted antigen-binding domain as well as seven novel synthetic controls of cell function that are collectively expected to overcome the unique challenges associated with treating solid tumours. These include a CXCR2 receptor to promote CAR T-cell trafficking to the site of the tumour, as well as chimeric TGFβ to redirect immunosuppressive signals in the tumour microenvironment.
In addition, FT825 is engineered to express a high-affinity, non-cleavable CD16a receptor. This is expected to improve the therapeutic outcome of FT825 when administered in combination with HER-2 targeting monoclonal antibody (mAb) therapy, since CD16a will bind to mAb-coated cells, leading to antibody-dependent cell-mediated cytotoxicity (ADCC). However, wild-type CD16a is enzymatically cleaved upon stimulation. Research undertaken by Fate Therapeutics and researchers at the University of California San Diego and University of Minnesota demonstrated that the introduction of a specific point mutation in CD16a (hnCD16) prevents this activation-induced surface cleavage (1), and the company has shown in pre-clinical studies that co-activation of FT825's HER2-targeted CAR and hnCD16 Fc receptor through combination with mAb therapy led to enhanced anti-tumour activity in animal models.
The inaugural CRISPR Medicine Conference is getting closer!
The world's first CRISPR Medicine Conference is taking place in Copenhagen, Denmark in April 2024.
The event will bring European CRISPR research to the forefront, with 6 main tracks that cover the most important topics in the therapeutic gene-editing field: Tools, Delivery, Safety, Functional Genomics, Standards and Regulations, Pre-clinical Research and Clinical Trials.
Our keynote speaker is Luigi Naldini, Director of San Raffaele Telethon Institute for Gene Therapy (Italy)
Check out the incredible list of confirmed speakers here
See which companies, universities, research institutes and investors are attending here
Here are the important dates:
- January 12th, 2024: Early bird registration deadline
- January 24th, 2024: Abstract submission deadline
For registration details and more info, see here
The FT825/Ono-8250 trial
The Phase 1 trial of FT825 is designed to investigate a single dose of FT825 as monotherapy and in combination with mAb therapy in previously treated patients with advanced HER2-expressing solid tumours. The trial will encompass dose escalation and dose expansion portions, which are expected to evaluate safety, tolerability, pharmacokinetics and efficacy. Outcomes to be meaasured include overall response rate, duration of response and disease control rate.
According to a press release published this week by Fate Therapeutics, Fate will jointly develop and commercialise FT825/ONO-8250 with Ono Pharmaceutical in the United States and Europe, while Ono maintains exclusive development and commercialisation rights for the product candidate in the rest of the world. The companies are currently conducting pre-clinical development of an additional solid tumour programme targeting an undisclosed tumor-associated antigen.
Additional trials in cancer and lupus
In addition to the FT825 trial discussed here, Fate Therapeutics is sponsoring four clinical trials for various cancers and lupus. These include a Phase 1 trial for FT576, a BCMA-targeting CAR-natural killer (NK) cell therapy candidate, for the treatment of multiple myeloma, a Phase 1 trial for FT822, a CD19/41BB-dual targeting CAR-NK cell therapy for the treatment of B-cell lymphoma, and a Phase 1 trial for FT819, a CD19-targeting CAR T-cell therapy for ther treatment of B-cell cancers.
In July 2023, the FDA cleared Fate's IND application for FT819 for the treatment of systemic lupus erythmatous (SLE), which is the most prevalent form of lupus. According to a 3rd quarter 2023 company update, Phase 1 study startup is ongoing for FT819 in SLE.
We will share further updates from Fate Therapeutics and FT825/ONO-8250 as they emerge.
Check out our extensive collection of clinical trial updates and our clinical trial listing to learn more about ongoing clinical trials, and stay tuned for regular clinical and research updates.
References
- Zhu H, Blum RH, Bjordahl R, Gaidarova S, Rogers P, Lee TT, Abujarour R, Bonello GB, Wu J, Tsai PF, Miller JS, Walcheck B, Valamehr B, Kaufman DS. Pluripotent stem cell-derived NK cells with high-affinity noncleavable CD16a mediate improved antitumor activity. Blood. 2020 Feb 6;135(6):399-410.
To get more CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
ArticleNewsClinical News UpdatesCAR-TCRISPR-CasFate Therapeutics, Inc.
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.







