First Patient Is Dosed With Base Editor to Treat Familial Hypercholesterolemia. Interview: Sekar Kathiresan, CEO Verve Therapeutics
Do you plan to organise a webinar?
Why not use the CMN platform and get exposed to the global gene editing community.
More details here.

Heterozygous familial hypercholesterolemia (HeFH) is a genetic disorder characterised by elevated levels of low-density lipoprotein cholesterol (LDL-C), which results in a greater risk of developing heart disease. The disorder may arise through mutations in several different cholesterol-regulating genes, and manifests as an inability to remove the excess cholesterol that accumulates in the blood, leading to heart disease.
HeFH affects approximately 1 in 250 individuals worldwide, making it one of the most common genetic disorders.
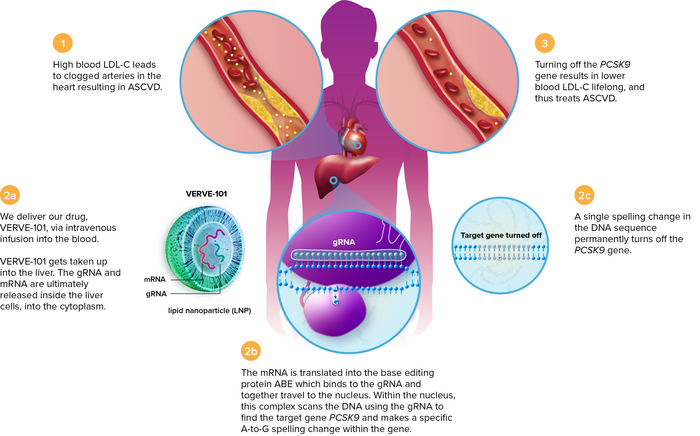
U.S. company Verve Therapeutics aims to treat HeFH using a specialised base-editing gene editor, which can turn off a cholesterol-raising gene in the liver of individuals with HeFH to permanently reduce their LDL-C levels to a healthy range.
The investigational therapy is called VERVE-101 and the first patient was recently dosed as part of a Phase 1 clinical trial in New Zealand.
Here, Verve Therapeutics’ CEO Sekar Kathiresan has kindly offered to speak with us about the therapeutic strategy behind VERVE-101, and what this could mean for individuals living with HeFH and other types of cardiovascular disease.
- Sekar, thanks for taking the time out to speak with us. Maybe you could start by telling us about the current treatment landscape for FH?
FH is a morbid genetic disease characterised by lifelong high levels of the bad cholesterol, LDL cholesterol or LDL-C, and early heart attacks. This means that susceptible individuals often experience symptoms of heart disease in their 30s, 40s, and 50s.
The primary treatment strategy is to lower LDL levels as much as possible, for as long as possible. This involves lifelong management of LDL levels through a combination of lifestyle interventions and cholesterol-lowering medications.. However, despite the availability of these interventions, research shows that fewer than 3% of patients being treated for FH worldwide are actually at their LDL goal.
Mimicking a natural variant
So, how will a base editor address this problem?
Our ultimate goal was to lower LDL levels as much as possible, for as long as possible. When it comes to LDL levels, extensive research has shown that there's no level that is too low. There are people walking around with cholesterol-raising genes that are naturally “turned off”. These naturally-occurring variants actually render these individuals resistant to heart disease. It is the effect of one of these variants that we are mimicking with VERVE-101, by using a base editor to turn off a cholesterol-raising gene called PCSK9.
“Research shows that fewer than 3% of patients being treated for FH worldwide are actually at their LDL goal.”Sekar Kathiresan
- OK, and how exactly does VERVE-101 work?
VERVE-101 is a one-time infusion consisting of two components. One is the adenine base editor (ABE), that changes an A to a G with incredible precision within the genome. Then, we have designed and constructed a guide RNA to take the base editor to the precise desired location in the PCSK9 gene. Once the base editor has made a base change from A to G at this specific position in the PCSK9 gene, the gene is permanently turned off, leading to lower LDL cholesterol levels.
Lipid nanoparticle delivery
Delivery continues to be one of the biggest challenges in developing gene-editing therapies, and getting them to where they need to be in the body. Can you tell us exactly how VERVE-101 reaches the liver?
The base editor is delivered as mRNA, and this is packaged along with the specific PCSK9-targeting guide RNA in a little fat bubble called a lipid nanoparticle. The lipid nanoparticle is infused intravenously into the bloodstream of a patient over approximately 60 minutes.
Once in the bloodstream, the lipid nanoparticle is taken up by liver cells through a receptor-mediated uptake mechanism, and once inside a liver cell, the contents of that fat bubble are released into the cytoplasm.
And, how much of VERVE-101 reaches the liver? Are there any concerns about off-target effects?
Almost all of it reaches the liver. We do see some biodistribution in two other tissues, the spleen and adrenal gland, but there’s a very low level of uptake in those two tissues. As for off-target effects, a lot of intricate screening was performed to find a spot on the PCSK9 gene where there was no bystander editing, i.e., editing of other nearby As. In addition, we do not observe any off-target editing at any of more than 6,000 sites tested in primary human liver cells treated with drug.
In addition, within 48 hours following infusion, our drug product, i.e., the mRNA and the guide RNA is almost all gone. Within a few days of infusion, the lipid nanoparticle is degraded. All you're left with is the edit, and the fact that the PCSK9 gene is turned off permanently.
Pre-clinical findings
- Can you tell us about the pre-clinical studies for VERVE-101? What disease models were used and what results led to human trials?
Our pre-clinical studies involved the use of cells, mice and monkeys treated with VERVE-101. A lot of our work was carried out with monkeys given how extremely comparable the monkey model is to humans.
The biology of the lipid nanoparticle that delivers the drug has translated really well between monkey and human, whereas it translates considerably less well between mouse and human. In addition, the base editor mRNA has translated very well between monkey and human and the LDL biology is very similar between the two, whereas the LDL biology between mouse and human is not at all similar.
Lastly and very importantly in terms of gene editing, the DNA sequence that we want to change in the PCSK9 gene happens to be identical between monkey and human. Our studies revealed that VERVE-101 resulted in cholesterol lowering by 70%.
A one-and-done treatment
- I understand that VERVE-101 is being designed as a one-time curative treatment. What can you tell us about the durability of response in pre-clinical studies?
The longest interval monitored during pre-clinical studies was two years, and we found that the LDL levels were lowered for that entire interval. After the single dose, LDL levels were reduced by 70% at two weeks and remained 70% lower at two years. That is lower than any of the current treatments have achieved so far, after just one dose of VERVE-101.
- The patient who has received the first dose of VERVE-101, had this individual already suffered a heart attack?
Yes. The inclusion criteria for the Phase 1 trial is genetically proven FH, they have high LDL, and they've already had a heart attack.
Expanding treatment to all cardiovascular diseases
- Given that FH can arise through mutations in multiple different genes, is VERVE-101 expected to benefit all individuals suffering from this disease, or will it depend in any way on their causative mutation?
It'll benefit everybody. PCSK9 is a gene that raises LDL cholesterol in everybody, including FH patients. Therefore, using VERVE-101 to turn off PCSK9 would provide therapeutic benefit to both FH patients and non-FH patients suffering from heart disease. This is how we hope to one day use VERVE-101 to treat all atheroscleotic cardiovascular disease as a whole, and even as a preventative step before the onset of heart disease.
There is a direct linear relationship between the degree of heart attack risk reduction and the degree of LDL lowering. For every 40 milligram/deciliter lowering of LDL cholesterol after a heart attack, you get a 22% reduction in risk of heart attack and death. Therefore, lowering LDL by 70%, as VERVE-101 has shown to do, massively decreases the risk of heart attack.
“For every 40 milligram/deciliter lowering of LDL cholesterol after a heart attack, you get a 22% reduction in risk of heart attack and death. Therefore, lowering LDL by 70%, as VERVE-101 has shown to do, massively decreases the risk of heart attack.”Sekar Kathiresan
- So, does Verve Therapeutics plan to use VERVE-101 for all types of heart disease in the future?
That is our vision for the company, yes. Starting with FH, we will use a genetic treatment for a genetic disease. Then, our goal is to expand the use of this medication, not just for FH patients, but the average atherosclerotic cardiovascular disease indication. Ultimately, this medicine could be used to prevent a first heart attack in at-risk individuals. Since the number one way to prevent a first heart attack is to reduce somebody's LDL as much as possible for as long as possible, VERVE-101 could essentially be used as a prophylactic treatment to prevent heart disease.
- The possibility to prevent heart attacks is very exciting. When young people have heart attacks, how routine is it to sequence their DNA to find out if they are genetically predisposed? And would it be easy to identify the at-risk people that might benefit from prophylactic treatment with VERVE-101?
I wouldn’t say sequencing is routinely done in that situation, but it is done. In terms of diagnosing FH, there are a couple of different ways to arrive at the diagnosis. You can use genetic testing to screen for mutations. Or, a diagnosis can be made purely on clinical grounds, based on family history, LDL levels or some physical exam findings.
However, the number one way is always to look at LDL levels. Individuals that have LDL levels over 190, which is about 5% of the U.S. population and 5% of Europe too, are at high risk of having a heart attack. These would be the individuals you might initially start with preventive therapy with VERVE-101, before a heart attack occurs.
Turning off spare genes
- Is Verve working on other in vivo therapeutic candidates?
We are working on other programmes focused on the liver. Specifically, our second programme targets a different gene called ANGPTL3. This gene is essentially very similar to PCSK9 in that it's a disease-causing gene that raises cholesterol. We identified people who naturally lack the gene and have very low levels of cholesterol and triglycerides. We're currently pursuing a one-and-done base-editing therapy to turn this gene off. We have some ideas for other genes that are similar to this, where turning them off could be beneficial. I think this is a concept that some people are a little bit puzzled by, this idea that you could have a gene that is a spare part.
- How far along is that programme?
Our guidance is that in the second half of this year, we will name a development candidate.
- There is currently no base-editing therapy on the market. When do you think VERVE-101 will be accessible to the public and how will it impact the future of treating heart disease?
The medicine will hopefully be available somewhere in 2028. The medicine itself, we believe will have a dramatic impact on this disease, because the disease is so poorly cared for at present. We think the answer is a one-time therapy that is effective and safe, that can dramatically and durably lower LDL cholesterol.
- So, when can we expect the first clinical readouts from this trial?
We are expecting initial results from the Phase 1 trial, which will include LDL lowering data, blood PCSK9 lowering data and safety data, sometime in 2023.
- Okay, we are looking forward to future updates about VERVE-101. Thank you for speaking with us today.
Sekar Kathiresan biography
Dr. Sekar Kathiresan is co-founder and CEO of Verve Therapeutics, a clinical-stage biotechnology company pioneering a new approach to the care of cardiovascular disease. Dr. Kathiresan is a cardiologist and scientist whose groundbreaking discoveries in human genetic mutations led him to co-found Verve Therapeutics with a vision to create a pipeline of single-course, gene editing therapies focused on addressing the root causes of this highly prevalent and life-threatening disease.
Prior to Verve, Dr. Kathiresan’s roles included director of the Massachusetts General Hospital Center for Genomic Medicine, director of the Cardiovascular Disease Initiative at the Broad Institute and professor of medicine at Harvard Medical School. For his research contributions, he was recognized by the American Heart Association with its highest scientific honor – a Distinguished Scientist Award.
This interview has been condensed and edited for clarity.
Billie Pang is a Biochemistry graduate and freelance science writer based in Ireland.
To get more of the CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
ArticleInterviewNewsClinical News UpdatesFamilial Hypercholesterolemia, FHBase editorsVerve Therapeutics, Inc.
CLINICAL TRIALS
Sponsors:
Wave Life Sciences Ltd.