Gene Correction With Cas9 Nickase Holds Promise for Rare Skin Disorder Junctional Epidermolysis Bullosa
CMN WEBINAR 21st Sept. 3:00 PM CEST
Gene Editing for Rare Skin Disorder Junctional Epidermolysis Bullosa
Register here

Type XVII collagen is crucial for maintaining stability between different skin layers, particularly between the dermis and epidermis. Mutations in COL17A1, the gene encoding collagen XVII, have been implicated in a debilitating hereditary form of epidermolysis bullosa (EB, see Fact Box).
Although gene correction using CRISPR-Cas9 has emerged as a promising strategy to treat genetic diseases, there are no methods to treat EB by restoring COL17A1 function.
In a recent study published in Molecular Therapy, a team of researchers led by Ulrich Koller at the Department of Dermatology and Allergology, Paracelsus Medical University Salzburg (Austria), developed a patient-specific proximal paired nicking method to reframe a homozygous 2-bp deletion in exon 52 of COL17A1, a mutation commonly found in patients with junctional epidermolysis bullosa (JEB).
Paired nicking-based COL17A1 editing led to COL17A1 reframing in primary JEB keratinocytes and three-dimensional (3D) skin models, restoring collagen XVII localisation and function.
»Our findings show for the first time that COL17A1 gene reframing via paired Cas9 nickases is a very efficient and safe option for the treatment of JEB and potentially other genetic defects caused by pathogenic frameshift mutations,« said Dr. Johannes Bischof, the first author of the study. »Our findings also demonstrate the superiority of Cas9-nickase-based targeting over wild-type Cas9-based strategies for gene reframing, which can be adapted for other diseases beyond EB,« he added.
Epidermolysis Bullosa
EB is a group of rare skin diseases, most of which are inherited. The lack of certain skin proteins makes the skin sensitive to friction or trauma, causing painful blisters. The hands and soles of the feet are particularly prone to pressure-related trauma, and scarring in these areas can affect the quality of life. JEB is a severe hereditary form of EB caused by pathogenic frameshift mutations in the genes encoding laminin-332, type XVII collagen, and integrin-a6b4. In patients with JEB, problems in attachment between the epidermis and basement membrane cause blistering in the top portion of the basement membrane. There are no curative therapies for JEB, and current treatments aim to alleviate symptoms and prevent complications associated with the disease, such as skin infections.
Bischof also noted that the high efficiency of protein restoration and the safety profile of the proximal paired nicking approach makes the research team confident that COL17A1 reframing in JEB keratinocytes using Cas9 nickase is a promising candidate method for clinical translation.
Targeting COL17A1 using Cas9 nickase
The use of ribonucleoprotein (RNP) complexes of recombinant Cas9 proteins and synthetic single guide RNA (sgRNAs) has many advantages over other gene-editing approaches. Because protein complexes have a defined half-life, they are only present and active in the nucleus of a cell for a certain amount of time, minimising safety concerns. »RNP complexes can be delivered efficiently into skin cells ex vivo via electroporation, making them ideal for our experiments,« Bischof explained.
The team used paired Cas9 nickase, a Cas9 nuclease variant with a D10A mutation, to reframe a common homozygous deletion in exon 52 of COL17A1 and to restore the correct reading frame in COL17A1 in keratinocytes from patients with JEB. They termed this approach “proximal paired nicking.” This strategy involves the use of two Cas9 nickase/sgRNA combinations that cleave opposite DNA strands and are close to each other at the mutation site (Figure 1).
Normal end-joining repair pathways are activated by the generation of this staggered DNA break, leading to nucleotide insertions and deletions (indels) at the mutation site, which result in a shift in the COL17A1 reading frame.
Elaborating further on the rationale behind using Cas9 nickase instead of wild-type Cas9 nuclease, Bischof noted: »Because the induction of double-strand breaks and their imperfect repair can be mutagenic to the cells, we opted to use Cas9 nickase, which cuts only one strand. These ‘nicks’ are much easier to repair with high fidelity, so they can reduce unwanted damage at off-target sites and are more suitable for clinical applications than wild-type Cas9.«
The team validated the selectivity and effectiveness of their approach using next-generation sequencing (NGS) of genomic DNA and RNA transcripts, western blot analysis, flow cytometry, and immunofluorescence staining in corrected keratinocytes and a 3D skin model.
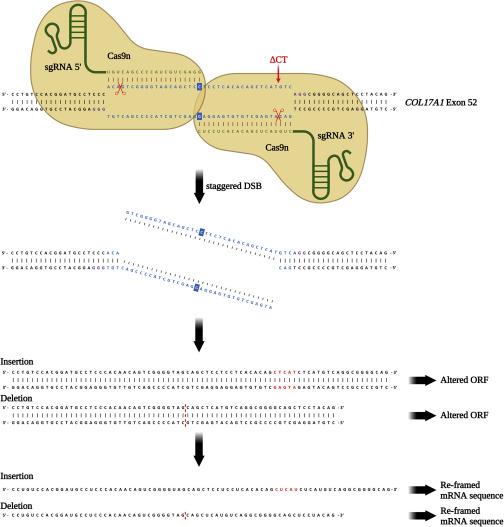
Paired nicking induces COL17A1 reframing in human keratinocytes
Electroporation of primary JEB keratinocytes with gRNAs and Cas9 nickase led to a high frequency (> 90%) of indels. In contrast, no indels were observed in wild-type keratinocytes. NGS analysis of genomic DNA showed that proximal paired nicking restored the COL17A1 reading frame in 28.3% of JEB keratinocytes.
To gain more insight into the types of indels induced by Cas9 nickase, the team performed NGS analysis of the transcriptomes of treated JEB keratinocytes. COL17A1 transcripts with 25- and 37-nt deletions were the most frequent, accounting for > 42% of edited transcripts.
The frequency of off-target indels with proximal paired nicking was low, ranging from 0% to 0.10%. In contrast, the frequency of off-target indels introduced by the wild-type Cas9 was up to 32.0%.
Paired nicking-mediated COL17A1 reframing restores collagen XVII expression and function in human keratinocytes
Flow cytometry analysis showed that paired nicking-based COL17A1 reframing restored the expression of full-length collagen XVII in 45.9% of the treated JEB keratinocytes.
Commenting on whether this percentage of JEB cells with functional collagen XVII would suffice for a clinically significant improvement in the skin condition, Bischof said: »There is evidence that physiological re-expression of functional COL17A1 may enhance the regeneration potential and longevity of the corrected cells by giving them an advantage over uncorrected cells. This would mean that we might not need to target every cell in a patient if, in the long run, the corrected cells outcompete the mutation-bearing cells.«
Immunofluorescence analysis confirmed that the expression of full-length collagen XVII was restored in JEB keratinocytes treated with gRNA/Cas9 nickase and showed that collagen XVII localised predominantly to the cell surface, where it was also localised in wild-type keratinocytes (Figure 2).
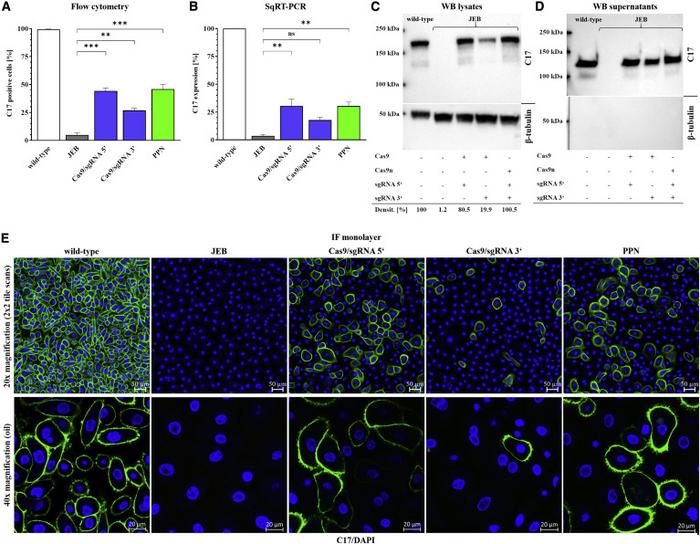
Inefficient keratinocyte adhesion to the basement membrane is central to EB pathogenesis. Cell adhesion analysis revealed that although ~75% of JEB primary keratinocytes adhered to laminin-332-coated dishes, 85–90% of corrected JEB keratinocytes and wild-type keratinocytes could adhere to the plates (p<0.05).
Consistent with their findings in primary keratinocytes, immunofluorescence staining revealed deposition of collagen XVII within the basal membrane zone between the epidermis and dermis in 3D skin equivalents generated using human JEB keratinocytes with paired nicking-based COL17A1 reframing.
»We demonstrated the correct membrane localisation of the restored protein in cells and correct deposition at the dermo-epidermal junction in 3D engineered skins, indicating correct protein function. Confirmation of these findings in a preclinical in vivo setting is still pending,« Bischof noted.
Challenges and future steps
Although this was the first study to show the feasibility of using proximal paired nicking to treat COL17A1-associated JEB by restoring collagen type XVII expression, Bischof said that they must confirm the superiority of this approach over recent gene replacement therapies.
Reflecting on the challenges they might face in advancing their method, Bischof said: »A key challenge is the lack of suitable models that would allow us to adequately assess long-term functional outcomes, such as longevity of the repair, long-term skin stability, level of clonality of the regenerated skin over time, wound healing, and proper repopulation with immune cells.«
The team plans to develop an appropriate in vivo model that would allow them to investigate the skin stability, long-term effects, and additional safety aspects of their proximal paired nicking approach. »Aside from this, we focus on how we can make the repair as perfect or ‘traceless’ as possible and improve the feasibility of an in vivo, rather than an ex vivo, therapy,« Bischof added.
»What’s clear is that there is still much to learn about type XVII collagen biology and how it influences keratinocyte stem cell dynamics and skin regeneration. Therefore, we favour a strategy that keeps regulation of gene expression under the control of its normal promoter rather than using gene replacement approaches,« he concluded.
Link to the original article in Molecular Therapy:
Paired nicking-mediated COL17A1 reframing for junctional epidermolysis bullosa.
Christos Evangelou, Ph.D., is a freelance medical writer and science communications consultant.
To get more of the CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
CLINICAL TRIALS
Sponsors:
Suzhou Maximum Bio-tech Co., Ltd.
Sponsors:
Zhejiang University