Gene-Edited T Cells Treat Leukaemia and Solid Tumours
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...

T cell receptor (TCR) cell therapy was once placed on the sidelines of onco-immunotherapy. Not only did researchers need to identify high-avidity, specific T cell receptors from the cells of healthy donors or patients, but they also had to generate complex genetic edits in T cell DNA to avoid the generation of mispaired receptors with unknown and potentially harmful specificities. With previous gene-editing technologies, this was an insurmountable challenge.
The discovery of CRISPR has led to a recent revival of TCR therapy, owing to its ability to perform multiple complex edits in human cells with relative ease.
Professor Chiara Bonini and her team at the Ospedale San Raffaele Scientific Institute, in collaboration with Intellia Therapeutics, recently discovered a TCR that is specific for Wilm’s tumour antigen 1 (WT1), the expression of which is strongly linked to the onset of cancer.
The group rationalised that the WT1-specific TCR could be knocked in to patient T cells in order to target a variety of cancers. In an article published in Science Translational Medicine, the researchers describe the use of CRISPR-Cas9 technology to create multiple edits in T cells to test various WT1-targeting TCRs, with promising tumour-killing results across a range of cancer types.
As first author and postdoctoral researcher Dr. Eliana Ruggiero puts it, the discovery of a high-avidity TCR for WT-1 is a major breakthrough for the field of TCR therapy:
»T cells expressing this receptor are able to eliminate all tested haematological and solid tumours.«

Hunting for T cell receptors
Bonini is a pioneer of TCR therapy, having previously published the original method for redirecting specificity of T cells via TCR gene editing. One of the challenges of TCR therapy that Bonini and Ruggiero describe is identifying candidate TCRs. Generating a shortlist of potentially relevant TCRs from healthy donors involves complex screening, isolation, and expansion procedures.
The TCRs identified in the study are specific to WT1, a transcription factor involved in cell differentiation and growth. Expression of WT1 is strongly linked to oncogenesis, with limited expression in healthy tissue. However, there are multiple WT1 epitopes, and previous studies have primarily focused on an epitope that is processed by the immunoproteasome, which is not operational in all tumour contexts.
Recognition of WT1 by TCRs is dependent on the presence of human leukocyte antigen (HLA), the human version of major histocompatibility complex (MHC). Of the three classes of HLAs in humans, only class I is present in all nucleated cells and this class has a key function in presenting antigens from infected or cancerous cells to cytotoxic CD8 T cells.
To create a TCR therapy that could treat a variety of cancer types, Ruggiero needed to find TCRs that could recognise multiple WT1-derived epitopes with varying HLA class I restrictions, and with high avidity. The chosen receptor also needed to be specific for WT1 epitopes that are naturally processed by the standard proteasome rather than the immunoproteasome, meaning that they are present in multiple cancers.
»The main challenge of the study was the identification of a candidate high-avidity tumor-specific TCR. Even though with our pipeline we have identified 19 different TCRs, we mainly focused on the HLA-class I restricted receptors and, among those, only one TCR was able to pass all the selection criteria we had established,« Ruggiero says.
The necessity of CRISPR editing for TCR therapy
Finding the right TCR is only the first piece of the puzzle in generating an effective TCR therapy. The next challenge is to create the necessary edits to the cells. If the TCR is identified from the cells of healthy donors – as was the case in this study - it must be knocked in to patient T cells to generate an autologous cell therapy, but other edits are also necessary.
The advent of CRISPR technology opened new doors for TCR therapy because it can be used to simultaneously create multiple edits in these primary cells - something previous technologies struggled to achieve.
The first edit necessary for TCR therapy is a knockout of the endogenous T cell receptor that is present in all T cells. This is because having both the transgenic TCR and the endogenous TCR present in the cells causes them to compete for signalling components. The two TCRs can mispair to form heterodimers that can result in autoimmunity and can also cause graft-versus-host disease (GvHD).
The endogenous TCR consists of alpha (TRAC) and beta (TRBC) chains, both of which must be targeted for complete knockout.
»Targeting the first exon of a molecule is preferred, in order to avoid the generation of a truncated protein that may retain functional activity. Our collaborators at Intellia therapeutics performed a thorough screening of several guides targeting TRAC and TRBC loci,« Ruggiero says.
After deleting the endogenous TCR, Ruggiero used lentiviral transduction to generate T cells containing different candidate TCRs specific to WT1. The next test was to pick the best-performing TCR.
The long-term success of any adoptive T cell therapy requires the activity of both CD4 (memory) and CD8 (cytotoxic/killer) T cell subsets. Memory T cells persist for long periods, patrolling in search of their target. This presents an additional challenge; anti-tumour TCRs that can activate both T cell subsets are very rare.
»HD1-TCR, our winner, has a greater functional avidity compared to the other tested receptors, and can activate both CD4 and CD8 T cells. I would say that the ability to retrieve from a healthy individual a receptor endowed with all these relevant features represents the highlight of the study,« Ruggiero remarks.
The team then performed targeted integration of the WT1-specific TCRs via CRISPR-Cas9, inserting the gene into the TCR α constant (TRAC) locus. The edited cells must then be expanded to produce the final autologous TCR cell therapy product for re-infusion into patients.
WT-1-specific TCR cells kill both leukaemia blasts and solid tumours
After identifying the winning candidate HD1-TCR, Ruggiero tested the HD1-TCR-edited T cells on a variety of cancers. Not only were the TCR cells able to kill acute myeloid leukaemia (AML) blasts in a mouse model, but they also display potent activity against acute lymphoblastic leukaemia (ALL) as well as glioblastoma tumours in mice (Figure 1).
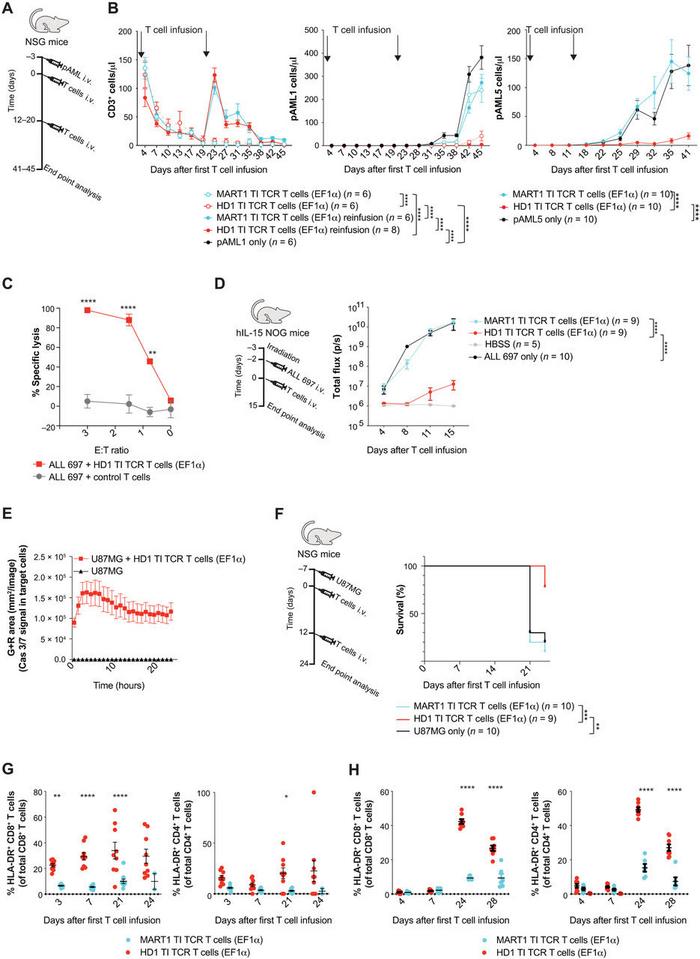
The HD1-TCR-edited T cells have an optimal safety profile, with no observed off-target CRISPR edits or cytotoxicity. There is also a reduced chance of tumour escape when targeting WT1 with TCR therapy, owing to the fact that WT1 is necessary for tumour survival. These considerations make HD1-TCR-edited cell therapy ideally positioned for clinical translation, something the team is currently working on with their collaborators at Intellia Therapeutics.
TCR therapy vs. CAR-T therapy: complementary medicines?
To non-specialists, TCR cell therapy and chimeric antigen receptor (CAR)-T cell therapy might sound quite similar. Both fall into the category of adoptive cell therapy (ACT) and are considered ‘living drugs’ - making a patient’s own T cells better able to fight cancer by engineering them to express particular receptors. However, the types of receptors used and their mode of action are very different.
TCRs are naturally occurring receptors that work via recognition of major histocompatibility complex (MHC) – known as human leukocyte antigen (HLA) in humans. HLA is a molecule that ‘presents’ antigens that are present in diseased cells to T cells. By presenting the relevant antigen, HLA tells the immune system that the cell is cancerous – a bit like forcibly pinning a nametag on the cancerous cell.
In contrast, the CAR-T approach uses engineered receptors that do not occur naturally. They are ‘chimeric’ because they contain two fused components: part of a T cell receptor and part of an antibody that is specific to the target cancer antigen. Unlike TCRs, CAR-T cells are highly specific only to target antigens that are expressed on the surface of cancer cells.
CARs do not recognise MHC/HLA, and so they cannot recognise intracellular antigens. As Bonini describes, this is one of the key advantages of TCR therapy over CAR-T.
»Cancer cells often have internal antigens, not surface antigens, so they cannot be recognised by CAR-T cells. But TCRs can recognise intracellular antigens,« Bonini elaborates.
TCRs also require significantly lower expression of the target antigen than CARs, and their binding affinity for the target molecule is in the micromolar range, compared to the nanomolar range of CARs. This allows TCR cells to infiltrate solid tumours, while CAR-T cells are typically halted at the surface.
CRISPR-Cas9 has accelerated the clinical translation of both TCR and CAR-T therapies by allowing researchers to perform multiple edits, including knockout of the endogenous TCR, targeted knock-in of the receptors, and other edits that increase the longevity and functionality of the cells.
Potential game-changer for solid cancer treatment
It often seems that TCR and CAR-T are opponent technologies in the race to treat cancer, however, Bonini says they should be considered as complementary therapies, with each providing what the other lacks as a comprehensive strategy to target cancer.
Notably, in the current study, the HD1-TCR-edited cells also display potent anti-tumour activity against glioblastoma, which is a solid cancer. T cell therapies for solid tumours have certainly lagged behind in terms of clinical translation compared to those for haematological malignancies, making this a game-changer for the field.
»We were really satisfied with the results obtained for solid tumors. We do know that WT1 is expressed on a variety of hematological and solid tumors, however it is not obvious that antigen expression correlates with tumour cell killing. The strength of the TCR we used enabled us to achieve this goal. It was great to see this receptor also working in a solid tumour context,« Ruggiero concludes.
Link to the original article in Science Translational Medicine:
To learn more about TCR cell therapy, you can read this 2020 review from the journal Cells, or watch Bonini’s 2016 TEDx talk discussing her work.
Rebecca Roberts, Ph.D. is a molecular biologist and science writer/communicator based in Queensland, Australia.
To get more of the CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
ArticleInterviewCancerSolid Tumor AdultSolid TumoursAdoptive T cell therapy (ACT)Intellia Therapeutics, Inc.
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.







