IND Update – Gene-Edited Allogeneic CAR-T Candidate for Multiple Myeloma
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...
Last week, Poseida Therapeutics, a clinical-stage biopharmaceutical company headquartered in San Diego, obtained investigational new drug (IND) clearance from the FDA for its first fully allogeneic CAR-T candidate for the treatment of relapsed or refractory multiple myeloma (MM).
The new candidate, called P-BCMA-ALLO1, is engineered to target the B cell maturation antigen (BCMA), which is primarily expressed by malignant and normal plasma cells and by some mature B cells, and which is the most widely studied target in CAR-T approaches to MM.
Poseida Therapeutics expects to dose the first patients with P-BCMA-ALLO1 later this year.
Cas-CLOVER and piggyBAC – a unique approach to CAR-T
P-BCMA-ALLO1 is developed using Poseida’s proprietary piggyBac® DNA Modification System and the hybrid Cas-CLOVER™ Site-Specific Gene Editing System.
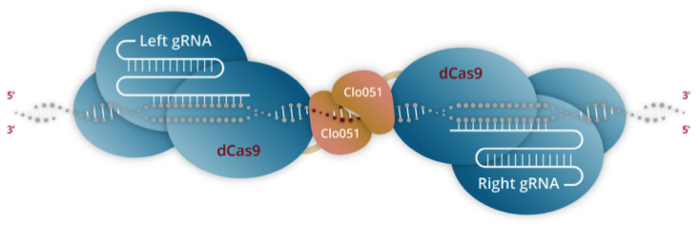
Cas-CLOVER combines the use of an enzymatically inactivated Cas9 endonuclease (dCas9) that is fused to Poseida’s proprietary dimeric nuclease, Clo051. In contrast to conventional CRISPR-Cas editing, two subunits of Clo051 – each of which is associated with target-specific gRNA – assemble to form a dimer at the target site. Cleavage of the target sequence only occurs upon dimerisation, which is believed to render Cas-CLOVER more precise than conventional CRISPR-Cas (Figure 1).
piggyBAC is a naturally occurring mobile genetic element that "cuts and pastes" or transposes DNA between vectors and chromosomes. Using Cas-CLOVER and piggyBac together, the genomes of healthy donor T cells are edited to express a BCMA-targeting CAR, whereby the Cas-CLOVER gene-editing reagents are delivered to the genome via piggyBAC.
The P-BCMA-ALLO1 trial
The trial for P-BCMA-ALLO1, which is not yet recruiting, is planned to be a Phase 1 open-label multi-center study in 40 (expected) adult participants with relapsed or refractory MM, and will follow a 3+3 study design of dose-escalating cohorts.
After a subject enrolls, allogeneic CAR-T cells will be administered as a single dose, following a standard chemotherapy-based conditioning regimen that is typically included in CAR-T treatment plans to destroy host bone marrow cells and reduce the risk of rejection of the infused therapeutic cells.
Trial participants will undergo serial measurements of safety, tolerability and response. Rimiducid may be administered as indicated to eliminate P-BCMA-ALLO1 from the body. Rimiducid can induce a safety switch (which is a gene that is engineered into the genome of P-BCMA-ALLO1 cells) that results in elimination of the CAR T cells from the patient if necessary.
The trial is estimated to start in October 2021 and the estimated study completion date, i.e., the date whereby data is available on the last study participant is estimated to be March 2038.
CAR-T cell therapy recap
CAR-T cell therapies comprise T cells that are engineered to express a so-called chimeric antigen receptor or CAR. A CAR is a synthetic transmembrane protein that contains two key domains: one that binds to the target antigen, most often a cell surface receptor that is abundant on diseased cells but not on healthy cells, and another that activates the T cell upon antigen recognition.
To develop a CAR-T cell therapy, cells from either the diseased individual (autologous therapy) or healthy donors (allogeneic therapy) are isolated and genetically-engineered to express a CAR. The most widely used CARs target CD70, CD19 or BCMA. Gene-edited CAR-T cell therapies are further engineered using a number of strategies in order to increase their persistence after infusion, improve safety and efficacy, and to mitigate the risk of relapse due to antigen loss in certain cancers. The engineered cells are then either transfused back into the individual to be treated or cryopreserved in off-the-shelf vials.
Is autologous CAR-T on the verge of extinction?
Poseida already has two autologous (i.e. patient-derived) CAR-T candidates in clinical trials for MM and prostate cancer, both of which have yielded extremely promising clinical results so far. These are engineered using piggyBAC technology to target the B cell maturation antigen (P-BCMA-101, Phase 2 candidate) or the prostate-specific membrane antigen (P-PSMA-101, Phase 1 candidate).
In a recent interview with CRISPR Medicine News, Poseida’s CEO Dr. Eric Ostertag MD PhD explained why increased stemness is key to effective allogeneic CAR-T cell therapies, how the company’s unique genome-editing approach gives a safey edge that has made it possible to run the P-BCMA-101 trial on a completely out-patient basis, and why allogeneic CAR-T therapies might actually render autologous counterparts extinct. You can read that interview here.
For a complete overview of current gene editing clinical trials, check out CRISPR Medicine News' Clinical Trials Database.
Tags
Articlein vivoElectroporationMultiple Myeloma, MMCancerCAR-TCas-CLOVERFDAPoseida TherapeuticsTrialsIND - Investigational New Drug
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.