Investigational New Drug (IND) Update
CRISPR-based therapies are advancing rapidly with new clinical trials emerging all the time.
Before any new therapy can enter a clinical trial, its developers must obtain clearance from the relevant regulatory bodies.
In the US, the clinical trial sponsor, which is usually the company or institute developing the new therapy, must submit an Investigational New Drug (IND) application to the Food and Drug Administration (FDA) detailing the preliminary data gathered from cellular models and animal studies. IND approval must be obtained before any clinical trial can take place. In Europe, preliminary safety data is included with a clinical trial application (CTA) to the European Medicines Agency (EMA).
Once approval is obtained for clinical testing by the relevant regulatory body i.e. FDA, EMA or otherwise, the programme may enter clinical development in that territory.
In this first IND update from CRISPR Medicine News, we look at three pre-clinical-stage CRISPR-engineered cell therapies that have obtained IND designation for the treatment of sickle cell disease (SCD), beta thalassemia, or acute lymphoblastic leukaemia (ALL).
EDIT-301: CRISPR-Engineered Red Blood Cells for Treatment of Sickle Cell Disease
Sickle cell disease is a group of disorders characterised by defective adult haemoglobin function, which compromises oxygen transportation in the blood leading to a range of complications that often reduce lifespan significantly. Foetal haemoglobin (HbF) is highly expressed and critical during foetal development, and is then rapidly suppressed early in life. Reactivation of HbF expression has emerged as an attractive strategy to treat the symptoms of SCD by compensating for the lack of functional adult haemoglobin by harnessing the inherent genetic ability to produce HbF.
EDIT-301 is a pre-clinical stage autologous therapy for the treatment of SCD. The new therapy is part of Editas Medicine's (US) ex vivo cell therapy pipeline. EDIT-301 is developed using a CRISPR-Cas12a ribonucleoprotein to enhance the HBG1/2 promoter region in the beta-globin locus of patient-derived red blood cells. Naturally-occurring HbF-inducing mutations at the HBG1/2 region support the clinical relevance of using gene editing to enhance the HBG1/2 promoter, which leads to an increase in the red blood cell levels of HbF.
Editas presented data at the 25th European Hematology Association meeting this summer demonstrating that red blood cells derived from EDIT-301 CD34+ cells exhibit a sustained in vivo increase in HbF in animal models.
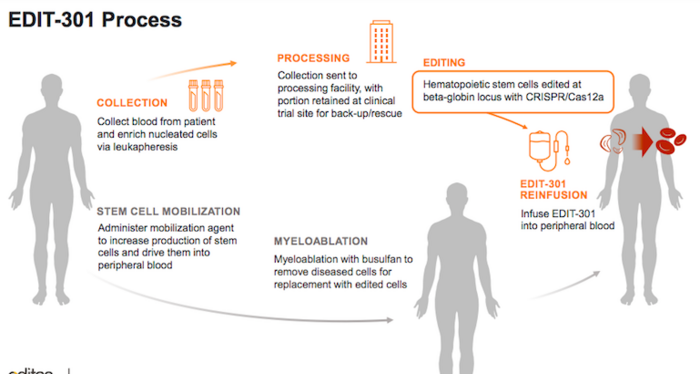
In August of this year, Editas obtained Rare Pediatric Disease Designation For EDIT-301 as a potentially best-in-class treatment for SCD in children from birth to 18 years.
ET-01: CRISPR-Engineered Stem Cell Therapy for Beta-Thalassemia
EdiGene, China is also developing an engineered cell therapy to increase HbF levels in red blood cells. The new ex vivo therapy, ET-01 is in development for the treatment of beta-thalassemia, a disease that shares some clinical overlap with SCD, but which is characterised by deficient rather than defective adult haemoglobin.
Beta-thalassemia arises from an imbalance in the beta globin chains of haemoglobin, which serve as the building blocks for mature and functional adult haemoglobin. Mutations in the beta globin (HBB) gene underly these imbalances, and given that there are at least 200 known disease-causing HBB mutations, most gene-editing strategies aim to increase HbF levels to compensate for the lack of haemoglobin rather than correct mutations in the HBB gene.
ET-01 is EdiGene’s most advanced pre-clinical candidate, and it is developed using CRISPR-Cas9 technology to disrupt and thereby reduce the activity of the B-cell lymphoma/leukaemia 11A (BCL11A) protein in patient-derived CD34+ cells. BCL11A is a negative regulator of HbF expression, thus its disruption increases HbF levels, and this is expected to lessen the clinical symptoms of beta-thalassemia.
EdiGene announced earlier this year that its IND application for ET-01 to the Center for Drug Evaluation (CDE) of China’s National Medical Products Administration (NMPA) had been accepted for patients with transfusion-dependent beta-thalassaemia (TDT), and the company is now preparing to move ET-01 into clinical development.
FT819: An Off-The-Shelf CAR T-Cell Therapy for Acute Lymphoblastic Leukaemia
Californian biopharmaceutical company Fate Therapeutics has a pipeline of induced pluripotent stem cell (iSPC)-derived cellular immunotherapies for cancer and immune disorders. One of these is FT819, an off-the-shelf CAR T-cell cancer immunotherapy candidate for the treatment of acute lymphoblastic leukaemia (ALL). FT819 is CRISPR-engineered to express a novel 1XX CAR that targets the CD19 B cell-specific cell surface antigen that is expressed in all B cell lineage malignancies.
Fate Therapeutics announced that it had obtained IND clearance from the FDA in July of this year, allowing it to proceed with the clinical development of FT819 for treatment of CD19+ cancers. FT819 is the first-ever CAR T-cell therapy to be derived from a clonal master iPSC line engineered with several first-of-kind features that are designed to improve the safety and efficacy of CAR T-cell therapy.
In FT819, the 1XX CAR is inserted into the T cell receptor alpha constant (TRAC) locus, which disrupts the native T cell receptor (TCR) locus while simultaneously placing the CAR under the regulatory control of the endogenous TCR promoter. Inserting a CAR at this location has previously been demonstrated to improve T cell function and potency in mouse models of ALL.
At last year's American Hemotology Association Annual Meeting, Fate Therapeutics presented encouraging in vivo preclinical data demonstrating that FT819 exhibits durable tumour control and extended survival in xenograft mouse models of ALL.
This was our first update about pre-clinical stage therapies based on gene editing. If you want to learn more about the clinical development path, you can check out our earlier explainer piece that covers the path from IND filing right through to Phase IV (post-market surveillance).
Tags
Articlein vivoAcute Lymphoblastic Leukemia, ALLBeta ThalassemiaSickle Cell Disease, SCDCAR-TGene therapyCRISPR-CasTrialsIND - Investigational New Drug
CLINICAL TRIALS
Sponsors:
Suzhou Maximum Bio-tech Co., Ltd.
Sponsors:
Zhejiang University







