Organ-Specific CRISPR With SORT Nanoparticles. Interview With Daniel Siegwart, University of Texas Southwestern Medical Center

CRISPR and mRNA-based protein replacement therapies have the potential to correct disease-causing mutations in any cell, tissue or organ type. Nanoparticles are a popular delivery vehicle for CRISPR-Cas and other gene editing modalities and are used in the first FDA-approved siRNA therapy (Onpattro) for the treatment of a rare peripheral nerve disease.
Despite the safety of nanoparticles and advances within gene editing technologies in general, it will be difficult to realise their full potential without having a robust way to perform gene editing on an organ- or cell- specific basis. Until recently, this hadn’t been possible, but a group at The University of Texas Southwestern Medical Center may have solved that issue with a novel strategy called ‘Selective ORgan Targeting (SORT)’.
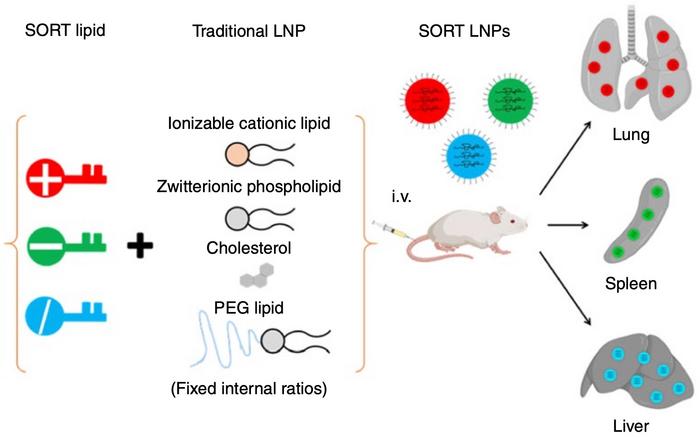
With SORT, a supplemental SORT molecule is added to established lipid nanoparticles that directs them to particular locations in the body. Using the modified SORT nanoparticles, the team, led by Daniel Siegwart PhD at The University of Texas Southwestern Medical Center, was able to specifically target a range of cell types within lung, spleen, and liver tissues. SORT nanoparticles are compatible with multiple gene editing techniques, including mRNA, CRISPR-Cas9 single guide RNA, and Cas9 ribonucleoprotein (RNP) complexes.
I caught up with Daniel Siegwart to hear more about the work, which was recently published in Nature Nanotechnology.
How it Works
- So how does the SORT strategy work?
Firstly, traditional lipid nanoparticles (LNPs) are tiny spheres (~100 nm in diameter) that are able to encapsulate nucleic acids inside their core. They can travel from the bloodstream to reach targeted organs such as the liver, lungs, and spleen. They are small enough to enter cells, and once inside a cell, molecules inside of the LNP become positively charged. This helps the LNP to disassemble and release its cargo, e.g., Cas9 mRNA and a target-specific guide RNA, inside the cell, where the cargo can then become an active drug.
SORT LNPs retain all of the molecules normally included in LNPs for encapsulation of nucleic acids. The extra SORT molecules alter the properties of the LNPs to control organ targeting and cell type specific uptake. We have determined that inclusion of SORT molecules directly alters where in the mouse the SORT LNPs go and which cell types are amenable to delivery. This occurs, at least in part, through adsorption of specific proteins in the blood. Each SORT LNP thereby forms a unique biological identity to mediate delivery to distinct organs. We will be publishing those results separately once they are biologically validated.
Potential for New Therapies
- You show that SORT LNPs can target a few select disease-relevant genes in an organ-specific way. Do you think that SORT can be applied to all genetic diseases?
Right now, we believe that SORT is applicable for the treatment of a wide range of genetic diseases, particularly those affecting the lungs, liver, spleen, and those related to the immune system and cancer. These are the organs and tissues that we have been specifically able to target with SORT LNPs. With respect to specific diseases, we have until now applied SORT to enable delivery of gene editing cargos for potential treatment of cardiovascular disease, Cystic Fibrosis, Primary Ciliary Dyskinesia (PCD), and Duchenne muscular dystrophy (DMD).
In the paper, we showed complete knockout of PCSK9 protein using SORT-nanoparticle delivery of Cas9 mRNA and a single guide RNA targeting PCSK9. PCSK9 is a clinically relevant target associated with Familial Hypercholesterolemia (FH) and Atherosclerotic Cardiovascular Disease (ASCVD). Although companies have developed and continue to develop siRNA-based treatments that temporarily decrease PCSK9, a CRISPR approach would potentially allow long-lasting decreases in PSCK9 and provide better treatment options for patients.
- SORT can be used to modify organ-specific gene expression. Did you apply SORT to correct any disese-causing mutations to wild type either in vitro or in animal models?
In another manuscript in revision, we demonstrated the use of SORT to correct a mutation in the dystrophin gene that causes Duchenne muscular dystrophy (DMD) in a mouse model of this disease. Moreover, The University of Texas Southwestern Medical Center has licensed the technology to ReCode Therapeutics, which is using these nanoparticles to develop therapies for Cystic Fibrosis and Primary Ciliary Dyskinesia (PCD). ReCode expects to file an Investigational New Drug Application (‘IND’) for both programs in 2021.
- Do you expect SORT to be relevant for all organs, or is there any anticipated limitation here?
We are currently testing the range of organs to which SORT can be applied. Based on our current understanding of the mechanism, which is driven by creation of an endogenous LNP identity in vivo, we are optimistic that SORT will be extendable to other tissues besides the ones we already examined.
Scaling Up and Expanding Applications
- What about scalability? Will it be easy to produce SORT nanoparticles on large scale?
Because SORT leverages extensive experience with LNPs, there are few differences between scaling SORT and any other LNP. Since LNPs are now used in many approaches, including in leading potential vaccines for SARS-CoV-2, we anticipate that companies such as ReCode will be able to scale SORT LNPs for preclinical studies and future clinical trials.
- Do you see other potential therapeutic uses for SORT LNPs besides delivery of gene-editing cargos?
We are exploring SORT technology for new cancer treatments. In one application, we are using SORT to improve gene editing in solid tumours, which can be synergistic with existing cancer therapies. In another application, we are working on in situ generation of CAR-T cells for immunotherapy, which could someday greatly simplify and reduce the costs of T cell immunotherapy.
As a side note, we have not found any size packaging limits of SORT nanoparticles so far, which highlights the potential of delivering a range of cargos to cells, and represents an possible advantage over other delivery strategies, for example, viral-based delivery of gene-editing components. We have also found that the transient nature of Cas9 deployment by mRNA and RNPs greatly reduces off target effects.
“When I started my independent lab in 2012, I wrote on the white board 5 major, long-standing challenges of our field. Every year, my lab members and I would review progress towards overcoming those goals. “Controlled nanoparticle delivery to organs other than the liver” was the hardest challenge that remained unsolved on the board year after year. Finally, about 4 years ago, we had a hypothesis of a potential approach that just might do it. That early idea became what we now call SORT.”
- So what's next?
CRISPR-Cas gene editing is an amazing advance that can potentially correct many disease-causing mutations with diverse cellular origin. However, issues around delivery of gene editing cargos present obstacles to clinical translation of CRISPR therapies. Nanoparticles have shown great potential in delivering such cargos to cells; however, current approaches have been limited to diseases of the liver. The central advance of this work was the discovery of a predictable strategy to selectively target specific cells and tissues outside of the liver.
SORT overcomes the common fate of nanoparticle-based drug delivery where “all nanoparticles go to the liver” and opens the door for high levels of mRNA-based protein replacement and CRISPR-Cas gene editing in specific cell populations in other tissues such as the lungs and spleen.
For the time being, we are working hard to gain deeper insights into the exact mechanism behind SORT, and we are excited about potentially expanding SORT to new tissues to treat an expanded set of diseases.
Link to original article in Nature Nanotechnology:
The Siegwart Lab
Daniel Siegwart’s lab is located in the Department of Biochemistry, Simmons Comprehensive Cancer Center, University of Texas Southwestern Medical Center, Dallas, TX, USA, and is headed by Dr. Daniel Siegwart, Associate Professor in Biochemistry. The lab’s central goal is to use materials chemistry and engineering to make a beneficial impact on human health. The group focuses on developing new materials for delivery of therapeutic RNAs and gene editing cargos, e.g., CRISPR-Cas9 components, to improve disease outcomes. Until now, the success of RNA-based therapies has been hampered by the difficulty of delivering these highly anionic biomacromolecular drugs into cells. The Siegwart lab is making advances in solving this problem through the development of synthetic carrier-based delivery strategies to mediate genomic editing in specific tissues for correction of genetic diseases.
Tags
ArticleInterviewNewsDeliveryLipid-based nanoparticleCRISPR-Cas
CLINICAL TRIALS
Sponsors:
Suzhou Maximum Bio-tech Co., Ltd.
Sponsors:
Zhejiang University







