Pre-clinical Development CRISPR Update
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...
EDIT-201: A CRISPR-Cas12a engineered Natural Killer Cell Therapy for Solid Tumours
Editas Medicine (Massachusets, US) is developing EDIT-201, an engineered allogeneic natural killer (NK) cell therapy for the treatment of solid cancers.
EDIT-201 is one of 6 ex vivo CRISPR cell medicines in Editas’ pipeline, and is being developed in collaboration with Sandhill Therapeutics (Texas, US). The collaboration combines Editas’ CRISPR-Cas technology with Sandhill’s BINATE™ production process, a novel universal donor technology to extract, isolate, and expand innate immune cells from the peripheral blood of healthy donors. These cells can then be engineered, e.g., using CRISPR-Cas, and developed as novel off-the-shelf cell therapies for the treatment of solid tumour cancers.
Natural Killer (NK) Cell Therapies
NK cells are a class of potent cytotoxic white blood cells with the ability to rapidly recognise and kill tumour cells. Recognition occurs via the expression of stress ligands or down-regulated self-antigen presentation via loss of major histocompatibility complex class I on tumour cells.
The unique ability to detect immune-evasive tumour cells has made NKs an attractive tool for novel immunotherapies against cancers, and in particular solid tumours. However, challenges exist in trafficking and infiltration of NK cells into tumour sites and their persistence and effectiveness is further hampered by low levels of the IL-15 cytokine (which is critical for NK survival) as well as immunosuppressive conditions within the tumour microenvironment, e.g, abundant transforming growth factor beta (TGF-β).
EDIT-201 Development
EDIT-201 is generated by a CRISPR-Cas12a-mediated double gene knockout in healthy donor-derived NK cells. Cas12a is delivered to donor cells as a ribonucleoprotein complex, which is programmed to disrupt the cytokine-inducible SH2-containing protein (CISH) gene - a negative regulator of IL-15 signalling - as well as the TGF-β receptor II (TGFBR2) gene. These knockouts are hypothesised to result in increased IL15 expression to support NK cell persistence as well as render the NK cells resistant to the suppressive effects of TGF-β.
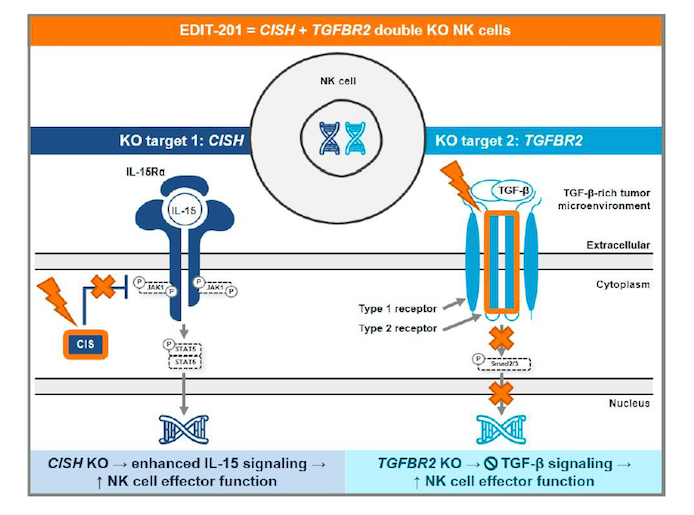
Editas Medicine presented promising pre-clinical data for EDIT-201 at The Society for Immunotherapy of Cancer (SITC) 35th Annual Meeting last month, demonstrating enhanced anti-tumour activity against multiple tumour spheroids and in an in vivo mouse model. The company plans to file an IND in the second half of 2021.
NTLA-5001: A CRISPR-Cas9 engineered T Cell Therapy for Acute Myeloid Leukaemia
NTLA-5001 is Intellia Therapeutics' (US) first engineered T cell receptor (TCR) therapy candidate and is being developed in collaboration with San Raffaele Hospital in Italy to treat AML patients regardless of cancer genotype.
Intellia is developing NTLA-5001 using patient-derived T cells, in which the TCR is engineered using CRISPR-Cas9 to target the Wilms’ Tumor 1 (WT1) antigen. WT1 is an intracellular protein that is often overexpressed in association with AML and other cancers. For now, the sole clinical target for NTLA-500 is AML, the most common form of leukaemia in adults which is rapidly fatal without treatment, but the company is also carrying out pre-clinical studies to investigate its potential for other WT1-positive solid tumors, such as ovarian cancer, glioblastoma, lung cancer and mesothelioma.
To develop NTLA-5001, Intellia deploys an undisclosed proprietary process to deliver nucleic acids into cells, which enables sequential CRISPR-Cas9 editing of 3 undisclosed genes without compromising on cell expansion potential and undesirable edits. The WT1-targeting TCR was engineered to disrupt the endogenous TCR locus in an attempt to produce a homogeneous TCR therapy product.

Intellia Therapeutics presented pre-clinical data for NTLA-5001 at Keystone Symposia’s Engineering the Genome in February of this year, demonstrating high editing efficiency, low toxicity, and specific and potent killing of WT1-expressing AML cells.
In May 2020, in a company press release in connection with 23rd Annual Meeting of the American Society of Gene and Cell Therapy, Intellia declared its plans to advance NTLA-5001 to clinical development next year.
Further Reading
For a complete overview of current gene editing clinical trials, check out CRISPR Medicine News' Clinical Trials Database.
You may also be interested in learning more about the clinical development path in our explainer piece that covers the path from IND filing right through to Phase IV (post-market surveillance).
Tags
Articlein vivoElectroporationAcute Myeloid Leukemia, AMLSolid TumoursCas12aCas9TrialsPre-clinical
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.







