Priming Therapy: Pre-clinical Applications of Prime Editing
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...
Hello, and welcome back to WeDoCRISPR!
In the last episode, we told you about Prime Editing, the next evolution of the CRISPR/Cas system! In this episode, we will discuss some examples of how Prime Editing is being used in the medical context. With this, we hope to make you understand why Prime Editing is a big deal and how versatile it is!
As we mentioned in the last episode, Prime Editing allows for a broader spectrum of single nucleotide changes compared to Base Editing, as well as the possibility of performing short insertions and deletions. It is not surprising that such technologies have already been tested in clinically relevant contexts, and today we shall tell you more about the clinical applications of Prime Editing! So buckle up!
Prime editing to treat liver diseases
Let’s start with Liver diseases!
By now you know that this is a classic! Liver conditions are a great test ground for gene editing applications for several reasons:
- They are often monogenic, so there is only one gene to blame (so only one problem to solve)
- The condition usually results in the accumulation of a toxic metabolite, so we can ideally cure it by just taking the faulty gene out, piece of cake.
- The liver is easier to reach – compared to other tissues – in vivo. Anything we put into the bloodstream will inevitably reach the liver, which is the main filter for our blood. Thus, the body’s own physiology can help us get CRISPR to the liver very efficiently!
For all these reasons, the liver represents a safe bet when comes to testing out new gene editing tools. Ask Intellia Therapeutics and Verve Therapeutics for example! They know what’s going on!
In the liver, prime editing has been tested for the treatment of diseases like hereditary tyrosinemia type 1 (HT1), a rare genetic disease caused by a defect in the enzyme fumarylacetoacetate (yes, that is indeed a word) hydrolase (FAH) which leads to toxic metabolite accumulation. There are several ways that this disease can be treated. For example, Intellia Therapeutics has developed a conventional knockout strategy targeting the ATTR gene. When ATTR is knocked out, the previous metabolic step, which produces the toxic fumarylacetoacetate, is prevented, fixing the problem before it begins.
Another option is to leverage the ability of Prime Editing to directly correct the disease-causing mutations in the FAH gene, which was shown to work greatly to treat HT1.
A similar approach has been used to correct mutations in FAH which are responsible for phenylketonuria (PKU). This has showed increased fitness and overall survival of the treated mice.

A similar approach has been taken for other diseases. For example, alpha-1 antitrypsin deficiency (A1ATD) is caused by a mutation in the SERPINA1 gene leading to the accumulation of a misfolded Alpha-1 antitrypsin protein. This mutation has also been targeted and corrected with prime editing.
Okay! So Prime Editing can be used in the liver. Where else? If you follow us for a while, you may already know that where there are Liver applications, there are Hematopoietic Stem Cells (HSCs).
Joining the party: prime editing can cure sickle cell disease
It is almost a given that if you have a working gene therapy technology, you are eventually going to wanna treat Sickle Cell Disease (SCD) with it. If you recall, in previous episodes we already mentioned how conventional knockout and Base Editing had been used to treat this disease. Remember Casgevy? You should, it was the first ever clinically approved CRISPR treatment! And of course, Prime Editing couldn’t miss the Sickle party! However, since you already know everything about this disease, we won’t go into much detail here.
Ok, let’s move to other tissues, like the eye!
Prime editing restores gene function
Now here things start getting more interesting, because we need to be a bit more subtle.
For example, Leber's congenital amaurosis (LCA) and Retinitis pigmentosa (RP) encompass a group of monogenic diseases that can lead to serious retinal degeneration. This is normally caused by dysfunctional genes in the cells of the retina. In those cases, knocking a gene out would not solve the problem, we need to restore the function of the mutated gene. And Prime Editing is the perfect tool for that! It allows us to correct point mutations, insertions and deletions that cause these diseases.
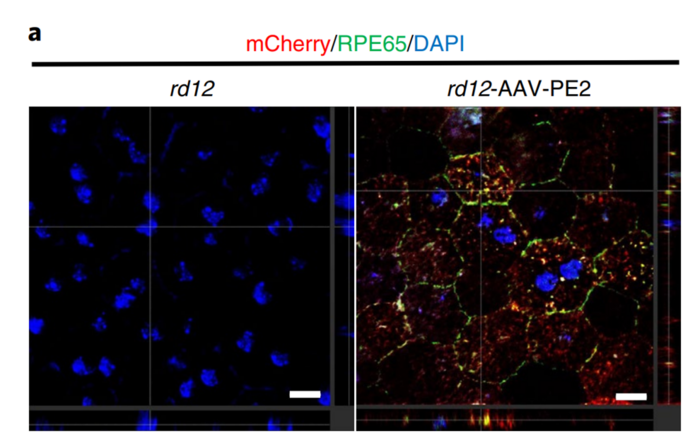
This is not only true for genetic conditions affecting the eye, but can also be applied in a myriad of other diseases, and it has. Some of the diseases that have been treated with Prime Editing in pre-clinical models include:
- Skeletal muscle diseases like Duchenne Muscular Dystrophy
- Neurodegenerative diseases like Alzheimer’s Disease
- Skin diseases like Epidermolisis Bullosa
- Systemic diseases like Cystic Fibrosis
Prime editing corrects mutations independently of HDR
It is amazing to think of how versatile Prime Editing has already proven to be! Of course, the major reason for its success is the possibility to bypass the double-strand break! This does not only have to do with the dangerous consequences of breaking up the DNA, but also with how we would normally correct a gene. If you remember, we mentioned in past episodes that standard CRISPR gene correction usually needs to use homology-directed recombination (HDR). However, HDR is not the favorite DNA repair pathway of the cell. Do you remember the other one? Indeed, non-homologous end joining (NHEJ)!
Let’s put it like this: you make a mess out of the DNA with CRISPR/Cas, the cell gets quite pissed and wants to tidy everything up quickly. Frankly speaking, the NHEJ pathway takes less time than HDR, and most of the time results in an accurate repair – even if sometimes mistakes can be made-, so why should the cell invest its time and effort in repairing the break via HDR?
Some cells don’t even bother with it! In fact, differentiated cells like muscle cells, skin cells, and others barely repair their DNA via HDR, making any applications of it highly unfeasible.
But Prime Editing does not need HDR, since it does not cause a DSB, does it? That’s the main reason why DNA modifications with Prime Editing are far more efficient in such cell types than conventional CRISPR/Cas approaches.
“But hold on! If Prime Editing can only perform small insertions of up to 100bp, how can it be preferable to CRISPR/Cas9?” we hear you saying. Glad that you asked, and we know that you were hoping to catch us by surprise. Too bad, we did our homework and the answer to your question lays in the next episode!
So, hold your breath and we will soon tell you how with small tricks it is possible to turn Prime Editing into a tool able to insert large DNA sequences, still without the need of DSB!
See you in the next episode of Making the Cut!!
To get more CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.