Removing the Brakes – Genomic Medicine in Experimental Clinical Practice
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...
For people with advanced melanoma skin cancer, a treatment utilizing immune cells derived from their own tumours holds great promise. Özcan Met, Associate Professor at Denmark’s National Center for Cancer Immune Therapy (CCIT–DK), is utilizing CRISPR–based gene editing to further improve this personalized, cell-based approach. His work highlights both what it takes to conduct complex clinical trials in an academic setting and why sustaining innovation beyond pilot studies remains challenging. These challenges are central to the motivation behind the emerging European Genomic Medicine Consortium (EGMEDC), which seeks to strengthen coordination, infrastructure, and long–term translational capacity across Europe.
Tumour–infiltrating lymphocyte (TIL) therapy is based on the observation that T–cells, one of the immune system’s key defences against cancer, can be isolated from metastatic melanoma tumours and turned into an effective treatment, as first shown in the mid-1990s.(1) »These immune cells have been ‘trained’ in the patient to recognize their melanoma but are then suppressed and exhausted by the tumour environment,« explains Özcan Met, Associate Professor and Group Leader / Head of the Cell Therapy Unit at the National Center for Cancer Immune Therapy (CCIT–DK), Herlev University Hospital, Copenhagen, Denmark.
To turn the lymphocytes into a cancer therapy, researchers collect patients’ T–cells from surgically removed tumours and reactivate and amplify them in a laboratory setting. When reintroduced to the patient by the hundreds of millions, these refreshed T–cells offer a renewed chance to overcome cancer’s defences.
A Meaningful Comeback
Technical challenges, combined with a shift in policy and funding priorities toward immune checkpoint inhibitors have kept TIL therapy largely out of the spotlight for many years, until gradual improvements in manufacturing led to approval of the first TIL therapy for advanced melanoma by the Food and Drug Administration (FDA) in 2024 (2).
Previously, CCIT–DK had reported the results of a phase 3 multicentre study with 168 advanced melanoma patients utilizing TIL therapy, in collaboration with the Netherlands Cancer Institute (3).
»Patients with advanced, metastatic melanoma are critically ill and have exhausted all standard treatment options, « says Özcan Met. »It was a significant step forward that almost half of this patient group responded to TIL therapy, with tumours shrinking and about one in five patients becoming disease free.«
In the United States, Lifileucel is offered by Iovance Biotherapeutics at a list price of $515,000 per treatment. At present, marketing authorisation has not been granted by the European Medicines Agency (EMA), and potential national reimbursement policies are still pending.
Nothing Goes Without Funding
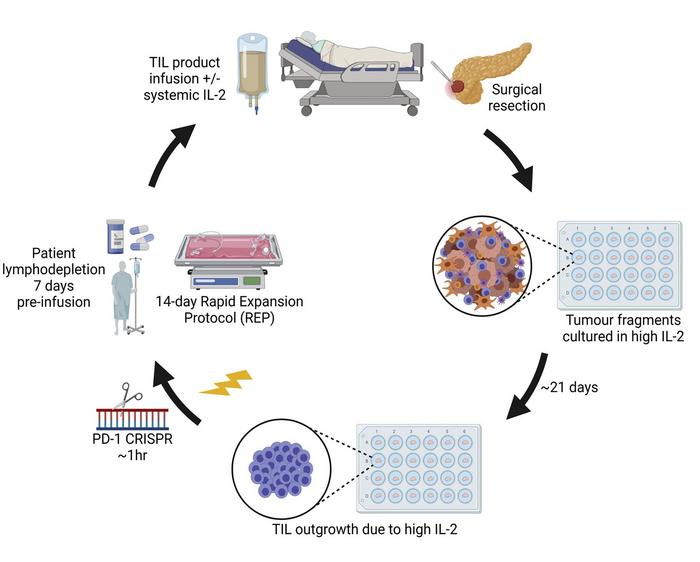
CCIT–DK is currently working on a pilot clinical trial that aims to further improve TIL therapy through CRISPR gene editing.
»We have done an extensive amount of preclinical work to show that CRISPR gene editing can be safely used to remove one of the T–cell ‘brakes’ that tumour cells exploit to suppress the anti-cancer activity of these immune cells. We use CRISPR-Cas9, formulated as a ribonucleoprotein complex and introduced to the cells by electroporation, to target and cut the PD–1 gene in the isolated TILs. It’s a step that only adds 1 hour to a manufacturing process that takes up to five weeks, and results in a nearly 90% reduction of PD-1 expression on the cell surface of the T–cells without any detectable off target effects. By eliminating the PD–1 brake, we aim to achieve higher and more durable response rates in patients.« (4)
“The pilot studies we run are made possible by competitive public and non-profit funding. When that funding cycle ends, it is difficult to warrant continuity of clinical progress.”Özcan Met, CCIT–DK
The essential financial support for this pilot trial of approximately one million euros was provided by the Danish Cancer Society, Novo Nordisk Foundation, and the Independent Research Fund Denmark. Met says: »You simply have to start small to demonstrate safety before broader clinical implementation becomes possible, as such the pilot study includes only ten patients. For now, our commitment lies with completing the pilot trial and publishing the outcomes. However, continuity is at risk for taking this work through the next phases of clinical research as additional funding or collaborative partners need to be found.«
The long-term scope for TIL therapy is not limited to melanoma. Özcan Met: »T–lymphocytes are present in other solid tumours including ovarian cancer, sarcoma, and renal cell carcinoma. But our current clinical protocols do not provide successful therapeutic means yet. We would like to change that, obviously.«
Avoiding the Complex CRISPR-Cas Patent Landscape in Clinical Trials
»An important point to make,« says Met, »is that this clinical trial fully qualifies as academic research. Yes, there are patients involved, but in practice it is not considered different to fundamental CRISPR experiments performed on the bench in a lab.« Patent law generally allows academic researchers to use patented technologies for non–commercial research, including investigator-initiated clinical studies, while licensing is required only when the work moves toward commercial use.
»Our CRISPR TIL trial is purely research based. There are no commercial incentives or partnerships. Once the trial has been completed, all results will be published in a scientific journal. I can’t be specific, but I can say we are looking forward to reporting the data.«
Building on Gained Experience in Clinical Manufacturing of Cell Therapies
CCIT–DK was established about two decades ago to develop and run small scale clinical trials to explore new cell-based treatments for patients. Met explains the aim is to first see if treatments are safe and note any improved outcomes.
Manufacturing clinical grade cell therapies require well defined and approved standard operating procedures in compliance with ‘Good Manufacturing Practice’ (GMP) rules. »One of our strengths is that we are building on experience, not so much as inventing something completely new, but a focus on refining existing protocols or equipment in a way that ensures continuity and standardisation.«
According to Met, creating an Advanced Cell Therapy is less like ‘doing an experiment from scratch’ and more like running a tightly controlled mini–factory for living cells, where every step is documented, contamination risks are engineered out, and each batch must prove through identity, purity, potency and safety testing that it is fit for treating a patient.
“Many potential CRISPR cell therapies share the same proven backbone, and when only small, preclinically validated changes are made, like swapping a guide RNA, it would help progress if there was more freedom to operate within that platform, while still fully evaluating what’s new and disease specific.”Özcan Met, CCIT–DK
Recognizing Common Ground Among Different Disease Treatments
Met is constantly looking to increase the clinical impact of the work performed at CCIT-DK. »Whether a therapy is aimed at treating cancer, immune dysfunction, or inherited disease, ex–vivo cell and gene therapies follow highly similar translational principles. Cells are harvested from the patient, modified or expanded under GMP conditions, and returned as a regulated medicinal product. The expertise developed around GMP manufacturing, quality control, and preparation of an Investigational Medicinal Product Dossier (IMPD) to apply for clinical trial approval at the EMA does not belong to a single indication, but forms a reusable framework that other academic groups in Europe can adopt when exploring gene editing and cell-based therapies in different disease areas.«
Accelerating Genomic Medicine Through Collaboration
For Met and his co–workers, it is crystal clear that collaboration is ‘a must’ in the field of advanced cell and gene therapies to drive clinical progress, even more so when rare or ultra-rare diseases are involved. International multicentre clinical trials are often the only way to enrol enough patients within a reasonable timeframe to generate robust data and reach meaningful clinical endpoints.
Met says: »Already, I am aligned with Northern European networks focused on Advanced Therapeutic Medicinal Products (ATMP), with the shared goal of translating promising experimental therapies from bench to bedside. However, I see clear added value in a closely knit consortium dedicated to genomic medicine – one that actively connects academic centres of expertise, patient representatives, regulatory authorities, and industry stakeholders.
»Knowledge exchange around Good Manufacturing Practice, the preparation of Investigational Medicinal Product Dossiers (IMPD), and, especially, collaborating outside of the box across diseases areas and national borders can really speed up the development and clinical introduction of advanced therapies made possible by groundbreaking genomic and cell technologies.«
Lifting the Brakes on Genomic Medicine in Europe – EGMEDC
In April 2026, at the upcoming CRISPR Medicine Conference (CRISPRMED26) in Copenhagen, a new non-profit initiative will officially be launched to bring a fragmented European genomic medicine landscape together to accelerate the clinical roll–out of genomic therapies: The European Genomic Medicine Consortium (EGMEDC).
Work emerging from leading academic expertise centres highlights both the strength and the fragility of innovation in genomic medicine. Academic hospitals are uniquely positioned to responsibly explore high–risk, high–impact approaches, such as gene-edited cell therapies, but these efforts often depend on short–term public or charitable funding. Without continuity, promising pilot trials risk stalling before they can benefit broader patient populations, regardless of their clinical merit.
The EGMEDC initiative aims to position itself as a bridge – a neutral framework for knowledge exchange, alignment, and capacity building. EGMEDC focuses on connecting stakeholders, academia, regulators, patient advocates, and industry, around shared translational challenges. By supporting shared platforms such as ex–vivo gene editing, GMP manufacturing, and regulatory preparation, the consortium aims to reduce duplication and raise the baseline level of expertise across centres in Europe.
From a policy, patient, and funding perspective, this infrastructure focus is critical. Advanced therapies will only deliver societal impact if access is not limited to a small number of highly specialized centres. Harmonizing practices, sharing standard operating procedures, and exchanging regulatory experience can help create a more level, sustainable, and affordable playing field, enabling more patients, in more regions, to benefit from genomic medicine.
Ultimately, lifting the brakes in genomic medicine is not just about scientific breakthroughs, but equally about pairing these innovations with coordinated infrastructure, shared standards, and a collective commitment to move forward together.
References
- Rosenberg, S.A., et al. (1994) Treatment of Patients With Metastatic Melanoma With Autologous Tumor-Infiltrating Lymphocytes and Interleukin 2, JNCI. 86, 1159–1166. https://doi.org/10.1093/jnci/86.15.1159
- Hu, L., et al. (2025) FDA Approval Summary: Lifileucel for Unresectable or Metastatic Melanoma Previously Treated with an Anti–PD-1–Based Immunotherapy. Clin Cancer Res. 31 (19): 4004–4009. https://doi.org/10.1158/1078-0432.CCR-25-0880.
- Rohaan, M.W., et al. (2022) Tumor-Infiltrating Lymphocyte Therapy or Ipilimumab in Advanced Melanoma. NEJM; 387: 2113-2125. DOI: 10.1056/NEJMoa2210233.
- Chamberlain, C.A., et al. (2022) Highly efficient PD-1-targeted CRISPR-Cas9 for tumor-infiltrating lymphocyte-based adoptive T cell therapy. Mol. Therapy, 24, p417-428. https://doi.org/10.1016/j.omto.2022.01.004.
Text by Henri van de Vrugt, PhD, Advisor to the European Genomic Medicine Consortium, New Haven Biosciences Consulting, For more information contact: henri@crisprmedicinenews.com
Disclosure of potential conflicts of interest:
HvdV is a freelance writer for CRISPR Medicine News and independent advisor to the European Genomic Medicine Consortium initiative. He is a founding partner of New Haven Biosciences Consulting, Connecticut, and CEO of Bio Avenues, Utrecht, the Netherlands.
Tags
ArticleNewsEGMEDCEGMEDCmemberTumour-infiltrating lymphocytes (TIL)
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.







