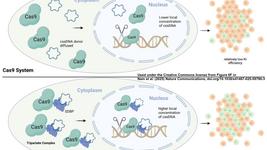
Safe and Precise CAR Integration
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...
In today’s episode, we complete this side chapter about using CRISPR/Cas with CAR-T.
In previous episodes, we saw how CRISPR can: i) help generate “off-the-shelf” CAR-T cells ii) make the CAR-T fearless and more powerful against cancer cells.
However, we are left with “How do we bring the CAR to the T cells in the first place”?
This question has been unanswered since the first episode, but today we shall answer it!
Producing CAR-T cells in the times of yore
In a time before CRISPR (B.C), integrating viral vectors were the main tool of gene and cell therapy. Particularly Lentiviruses.
We won’t get into details here. But in short, once a Lentivirus has entered the host cells, it releases its single-strand RNA which is then retro-transcribed to DNA. This new DNA strand, which will encode for the viral genes, is latter shuttled to the nucleus, where with the help of another viral component – the integrase – it is inserted into our genome.
And as the annoying roommate who keeps using our stuff to prepare his messy breakfast, the virus will use our replicating machinery to produce other copies of itself and spread the mess around.
By carefully studying this process, scientists came up with a way to render the virus replication incompetent and keep only the key elements driving Lentivirus infection and integration. This turned lentiviruses into a delivery tool, or a lentiviral vector. The coolest part is that, in this lentiviral vector, we can modify the single-strand RNA sequence so that, instead of integrating the information for the disease, we would introduce and integrate the coding sequence for the CAR!
Ironically, one of the most used lentiviral vectors derives from HIV. And what does HIV like to infect? Among its favorites on the menu are T cells. And where do we need the CAR the most? Yep, on the T cells.
Indeed, some of the currently FDA-approved CAR-T therapies like Kymriah use a lentiviral vector to deliver the CAR! After delivery, the CAR gene gets inserted into the T cell DNA and the CAR receptor can be produced. We have a CAR-T cell.
So why are we talking about CRISPR? What does it bring to the table?
CRISPR allows precise CAR-T integration
When talking about CRISPR, everything revolves around the possibility of targeting a specific location, enabled by the sgRNA part of the CRISPR system. Full stop.
Lentiviral vectors are a great tool, but they come with the limitation of us not being to control where they will land in our genome. It is like hiring a moving company to bring all your furniture to your new apartment. But they will just dump everything anywhere they find a spot and you will need to take care of the rest. And if you are unlucky things may get lost or damaged in the process.
The same happen when using integrating viral vectors.
And why is this concerning? Well, if the lentivirus lands in a coding sequence and alters it, we may have serious issues. We don’t want to sound like a drama queen screaming “think about the children!”, but yeah think what would happen if the sequence that was altered was encoding for a gene preventing you from getting cancer or another disease. That would not be fun.
Although this is a remote possibility, it may happen. And in fact, in the last months there was some fuss around the FDA issuing a warning regarding potential T-cell malignancies following CAR-T therapies. Thankfully, this has been reported only in a handful of patients – 20 – from the thousands treated with CAR-T cells. However, this has been nonetheless a call for attention and action on the safety of CAR-Ts.
And this is where CRISPR may help us! As we saw in episode 11, CRISPR can be used to integrate new information in the genome.
The second point is quite important. In fact, with Lentivirus we don’t just deliver the CAR sequence, but, since we don’t know where it will integrate, we have to accompany with a promoter that will drive its expression.
Conversely, with CRISPR we know exactly where the CAR sequence is going, and therefore we can insert it right after an endogenous promoter! One of the most used CRISPR strategies is to insert the CAR right next to the endogenous TRAC promoter, which is key for the expression of the natural T Cell Receptor (TRAC). By doing so, researchers have observed that the CAR did not only work as well as the ones generated with lentiviral vectors, but even better!
In fact, in this study the CRISPR-generated CAR T cells are also less prone to exhaustion and differentiation, making them more efficient at eradicating cancer. This approach also has the consequence of turning off the endogenous TCR promoter, thus if we KO B2M – as seen in episode 13 – we can generate in “Off-the-shelf” CAR-T cells too! Talk about two birds with one stone!
CAR-T production by CRISPR: a viable alternative?
Is CRISPR therefore going to take over the CAR-T production business? Currently several biotech’s are developing CRISPR-based CAR-T cells, with or without the use of viral vectors to deliver the CAR. Still, as we have seen in the past episodes, CRISPR is not exempted from risks, as knocking in a sequence requires the introduction of a DNA Double Stranded Break (DSB) which can lead to unwanted edits both at the target site and elsewhere in the genome (remember off-targets?). Like with all therapeutics, it all comes to benefits over risks. The low number of T cell malignancies makes lentiviral vectors still a precious tool to generate CAR T cells, but it is worth investigating other approaches that can make a therapy safer and safer.
And as a matter of fact, in the next episode, we will tell you how it is possible to make KI strategies safer by bypassing the need to introduce a DSB!
Stay tuned!
To get more CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.