The world’s first FDA-authorized CRISPR application. Interview: Rahul Dhanda, CEO Sherlock Biosciences.

In February this year about a month before the World Health Organization (WHO) declared COVID19 a pandemic Rahul Dhanda, CEO at Sherlock Biosciences, walked into the middle of the workspace. He stood in the centre and made a pivotal announcement: The company would now focus 100% on COVID19.
»I didn't get any resistance. I was expecting people to have a lot of questions, and what I was met with was just widespread enthusiasm. The team said this is what we're here for, this is what we want to do,« says Rahul Dhanda.
Three months later, Sherlock Biosciences announced the landmark accomplishment of getting the first-ever FDA-approved CRISPR tool: The SARS-CoV-2 diagnostic test received Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA). The company redesigned their foundational platform SHERLOCK (Specific High Sensitivity Enzymatic Reporter unLOCKing) to a diagnostic COVID19 test, that delivers results in about one hour, and is now set to play a crucial role in stopping the pandemic.
Here Rahul Dhanda has kindly agreed to an interview for an inside view of how it happened.
- Now, of course, there is a tremendous sense of urgency about stopping the COVID19 pandemic, but take us back in time to January, when the virus was just coming out of Wuhan, China?
So when the virus came out in January, we were closely monitoring it. It wasn't clear what the severity of it would be. It wasn't clear how large a pandemic it was going to be. But we were very concerned that this was something we really should be thinking about and working on, and we kept close to it.
- Did you begin to develop a test?
We started looking for SARS-CoV-2 sequences, and there are both publicly available soruces and those generated by some of our collaborators. And there is also actual material starting to be cultured by various organizations, so we continued on our path with standard development. Then sometime in February, we saw just how severe this was becoming, and we had to make a decision.
- Ok?
I was working with my board, and within 24 hours, I had a conversation with every board member independently. And they all felt very strongly that we should try to solve this, but solve it first and figure out what it means for the business later because this was at such a critical problem. What we ended up doing is taking that decision to the company and pivoting the company to be focused 100% on COVID19.
The diagnostic kit
The kit works by programming a CRISPR-Cas13 complex to detect two genetic sequences specific to the SARS-CoV-2 genome. When the signatures are found, the Cas13 enzyme releases promiscuous cleavage activity, which cuts a quenched fluorescent RNA reporter and releases a fluorescent signal. The signal is detected using a plate reader. A positive control let researchers know that they had an appropriate reaction. Results are delivered in approximately one hour and in Sherlock's hands (about 2,000 tests) it is 100% specific meaning no false positives. In the clinical validations studies, it was also 100% sensitive.
Sherlock Biosciences is also developing a simple strip test like a home pregnancy test that requires no instrumentation, but the FDA-approved test requires a lab with the ability to amplify RNA and a plate reader, and is designed for use in high-volume CLIA laboratories and hospitals.
This is what we came to do
- Tell me a bit more about the sentiment in the company. I mean you are developing these CRISPR tests to detect pathogens more generally, and then suddenly there's a global pandemic rising, and an urgency that must affect everybody?
Yes. I think the specific sentiment is that everyone who joined Sherlock did so because they feel like diagnostics can make a much bigger impact on broader health care globally than just within the segment. What is so incredibly important to the team is the meaning in the work they're doing. The big vision is one they had, but to be able to relate to that big vision to a very specific application and product made them feel like they were doing what we are here to do. I think it was on everyone's radar already.
- So everyone was already wondering whether to do it?
We have members of the team with connections to Wuhan, and others whose family members are on the front line. We also have one team member who splits his time between one of the major hospitals in Boston and Sherlock, and he's on the front lines. So everybody had a close contact or a teammate at the very least, who was going to be called into one aspect of this effort to defeat the pandemic.
“we know what we came to do, and we did it.”
But even in the absence of those connections, the broader commitment to seeing the technology through to a major impact is what drove everybody.
I think, if we had not done it, we'd all be sitting here today feeling very, very badly about that decision. Instead, we're all sitting here today saying, you know, we know what we came to do, and we did it.
In three months time
- Great, everybody came together and what happened then?
So in just a very short amount of time - days - we developed an assay. We then felt that we had to find the right partner to scale it and commercialize it.
But then the FDA put out guidance that describes this emergency use authorization path, and that would allow us to develop it ourselves under the right structure.
It was mid-March, and so we had to set up an infrastructure that didn't exist within Sherlock - to have a level of quality control around everything, standardize protocols and essentially create the documentation necessary to be a real product organization.
We launched immediately into our development plan and ended up getting through all of our work by the middle of April. We submitted, and by May, we had approval.
Initially about 30,000 tests
- That's great, and when will the test be out there?
Right now we're still in manufacturing, and we expect in the next few weeks we're going to announce to give all the details. Right now, we are working on getting the products manufactured and launched. We will launch probably with something like 30,000 tests available, and then if we need to scale up later, we will scale up.
Our goal is to provide the volume that the market needs, and I'm optimistic that the tests they want out there in the market will be Sherlock, and I'm optimistic that the number they'll need is going to decrease. I hope that we get our hands around this pandemic.
- And how will this test make a difference?
I think what we'll see with a test like this is an ability to bring accuracy to the patients in a rapid timeframe of approximately one hour. With those results, you can make a lot of decisions, not just for how you deal with that patient, but also how you deal with the overall burden of health care, because of the higher throughput of patients that you're going to have to test. So the more you can do with high accuracy, the fewer tests that you have to repeat and the faster you make decisions.
Every one of those patients is going to have reliable answers that can both be used to manage a patient’s disease, as well as enable physicians to handle and manage entire populations with disease.
- So, for example, a patient having symptoms will get the test, and if the result is positive, then very quickly can self-isolate, and stop spreading the disease?
Exactly.
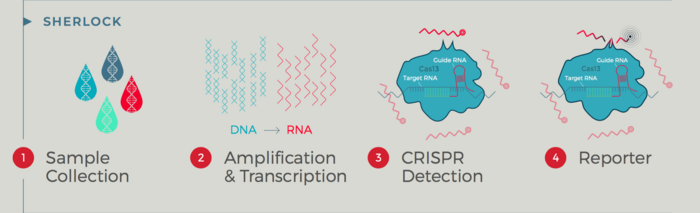
It's a diagnostic not a therapeutic
- So everyone agrees testing is critical to stopping the pandemic, but can you put some words on and how important this test is not to the world, but your company?
I'd be happy to. I think the best way to phrase it is that CRISPR for the longest time, has demonstrated incredible robustness and an equal promise. And at Sherlock, we are committed to bringing this through to being the most effective tool it could be. For that to happen, it isn't just about driving the R&D process, it's also about getting that product to the people who need it.
“The first real use of CRISPR is not a therapeutic; instead it's a diagnostic, and that is historic.”
Two important points are the historical moment where CRISPR has been authorized by this emergency use authorization pathway for use by the FDA. The first real use of CRISPR is not a therapeutic; instead it's a diagnostic, and that is historic.
And for the company, it validates that we are a mature player that knows how to bring a product to market, and can do all of the things we promise. We are focusing on the impact, and it is very validating for us that while many people are trying to work with CRISPR on research projects inside and outside of industry, Sherlock is the one that's making an impact. And that's what we're here for.
Ready for future pandemics
- And looking ahead the new diagnostics are going to help fight the current pandemic, but it will also be there to stop the next outbreak before it becomes a pandemic?
I think that that's true. What we've proved here at Sherlock is that the technology, CRISPR, is a highly responsive and deployable tech that even a company less than a year old can pick it up and bring a product to market.
That speaks to the team and also speaks to the technology.
I think in the future when a rapid response is needed - both the technology and the company, are going to be ready to get ahead of it. This time we were caught off guard because we had not built an organization to address it. But we're very proud that we rallied appropriately to address it.
In the future, we will have far more infrastructure and capability in place.
And while we hope we'll never have to deploy that for a pandemic, we will be ready, and we will address it.
Sherlock Biosciences
Sherlock Biosciences was founded in 2019 and is headquartered in Cambridge, Massachusetts, United States. It is an engineering biology company developing CRISPR-based methods to detect and quantify specific genetic sequences. The SHERLOCK assay was developed in the lab of Feng Zhang, who is one of nine co-founders.
Tags
ArticleInsightInterviewDiagnosticsCOVID-19Cas13aSherlock Biosciences, Inc.Approved (FDA)
CLINICAL TRIALS
Sponsors:
Suzhou Maximum Bio-tech Co., Ltd.
Sponsors:
Zhejiang University







