Tune Therapeutics: Can Epigenome Editing Cure Hepatitis B?

November of 2023 saw US startup Tune Therapeutics pull back the curtain on a major pre-clinical programme: a potential cure for chronic hepatitis B. Developed using Tune’s TEMPO platform, TUNE-401 aims to epigenetically silence the hepatitis B virus (HBV) without creating double-stranded breaks.
Dr. Brian Cosgrove, bioengineer and principal scientist at Tune, spoke about the company’s philosophy and science, the intricacies of HBV, and the TUNE-401 programme in a recent interview with CMN. Cosgrove highlighted the potential of TUNE-401 to offer a much-needed functional cure for HBV:
»This is an epigenetically regulated virus. So, let's come in with a tool to break that regulation.«
Genetic tuning with the TEMPO platform
Cosgrove explained that Tune Therapeutics arose from the idea that many meaningful phenotypic changes are caused by the precise tuning of gene expression levels, rather than genes being switched on or off. The company’s TEMPO platform employs CRISPR as an epigenome-editing tool, using catalytically-dead Cas9 (dCas9) fused to epigenetic effectors to reprogramme the epigenome in a site-specific manner.
The company emphasises TEMPO’s ability to activate, re-activate, and repress target genes with high levels of specificity, creating lasting effects without the concerns associated with traditional CRISPR-based gene editing - mutagenesis, translocations, and off-target editing, for example.
TEMPO is also able to perform multiplexed editing, making it well-equipped for the treatment of complex diseases that are caused by changes in more than one gene, including many cardiovascular conditions, cancer, and neurodegenerative disorders.
»We have these amazing epigenetic editing tools, and there's a lot of expertise and drive now to get some of these tools into the clinic and start to change the lives of the people that could really benefit from this,« Cosgrove said.
Perhaps counterintuitively, Tune’s first target does not require the fine-tuning of gene expression promoted on the company’s website, but rather the complete repression of the viral genome. Cosgrove suggested that HBV won’t be the only infectious disease target for the TEMPO platform going forward:
»This idea could really be applied to a lot different latent viruses, and especially latent DNA viruses,« Cosgrove commented.
An interesting virus: integration of HBV and ‘Franken-chromatin’
Transmitted via blood and bodily fluids, HBV causes both acute and chronic infections of the liver; chronic HBV can result in death from cirrhosis or primary liver cancer (hepatocellular carcinoma, HCC).
According to the WHO, in 2019 almost 300 million people were living with chronic hepatitis B worldwide, and it caused around 820,000 deaths. Despite the availability of a safe and effective vaccine, there are an estimated 1.5 million new HBV infections each year, primarily in the Western Pacific and African regions.
Cosgrove said the complicated and intriguing nature of the virus is what drew him and his colleagues to work on HBV. A small (3.2 kilobase pairs) DNA prototype virus belonging to the family Hepadnaviridae, HBV is classified into 10 different genotypes and possesses some unusual features.
»The HBV genome is partly single- and partly double-stranded,« said Cosgrove, who goes on to continue: »Once hepatocytes are infected, [the single-stranded part] gets ‘repaired’ by the host cell nuclei, and creates this circular, double-stranded mini-chromosome.«
These ‘mini-chromosomes’ are known as covalently closed circular DNAs (cccDNAs), and serve as the reservoir of new viral particles. Owing to a level of resistance to antivirals and the host immune system, these viral factories can persist for many years in patients, causing chronic hepatitis B.
Despite not being a retrovirus, HBV also manages to integrate into the genome of human hepatocytes over time. When an infected cell experiences a double-stranded break in its nuclear DNA, it will repair the lesion, often unintentionally using pieces of the cccDNAs for repair if there is slight homology on either end of the break. Regardless of chronic infection status, this integration of the virus into the human genome (intDNA) further complicates the treatment of hepatitis B.
While chronic hepatitis B infection can be treated with nucleoside analogues (reverse transcriptase inhibitors) that inhibit viral replication, the cccDNA persists in infected cells; instead of offering a functional cure, these medications simply aim to slow the progression of cirrhosis and reduce the likelihood of HCC.
The most interesting thing about HBV, Cosgrove said, is the epigenetic regulation of the cccDNA form of the virus.
»What’s weird is that this Franken-chromatin forms, that’s half human and half viral. To us, that was particularly fascinating – the rules that apply to native chromatin and gene regulation aren’t necessarily true for this viral mini-chromosome,« he explained.
TUNE-401: Putting HBV on Mute
A true functional cure for HBV would require the permanent repression of both forms of the virus in hepatocytes: intDNA and cccDNA. TUNE-401’s approach to this problem is to mimic a rare biological phenomenon observed in certain chronic HBV patients.
»Some patients can sporadically control the virus by putting on epigenetic marks to try to shut it down,« said Cosgrove. »But it takes many, many years on antivirals, and it's very random and stochastic. Our idea was that we could programme in these repressive epigenetic phenotypes, shut down the virus, and produce a true functional cure.«
TUNE-401 uses dCas9 fused to two effector domains – a methyltransferase and another epigenetic repressor – that work in concert to exploit a weakness in the epigenetic regulation of HBV. Delivered via lipid nanoparticles (LNPs), the candidate contains an mRNA sequence encoding the epigenetic repressors and a guide RNA sequence targeting HBV.
The target is a master controller sequence that is conserved between the intDNA and cccDNA of the virus across all known genotypes. By remodelling that specific viral locus and programming in a repressive state through DNA methylation and heterochromatin formation, TUNE-401 can inactivate all forms the virus.
»We have a single transient delivery of some of these tools, delivered by an LNP, they remodel this epigenetic locus, and then we're left with a really lasting, durable response. That's something that the field has been lacking for a while,« Cosgrove remarked.
Tune’s proof-of-concept research for TUNE-401 was performed in the Hep3B cell line, which is derived from male hepatocellular carcinoma and carries integrated HBV (see Figure 1 below). Further in vitro testing was performed in primary cells, followed by in vivo validation in humanised mice.
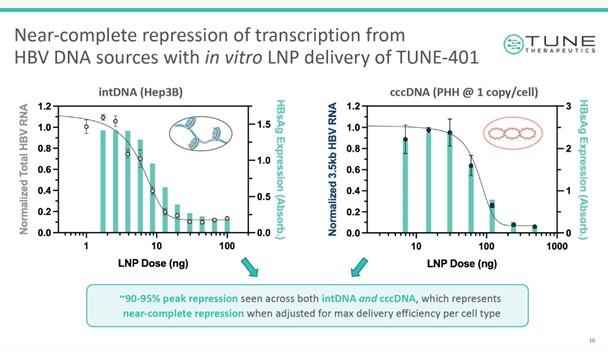
Durable responses in mice and upcoming human trials
Tune are now advancing toward IND filing for TUNE-401 and presented compelling pre-clinical data for the programme at the recent HepDART hepatology conference in Cabo San Lucas, Mexico. Because HBV does not infect the non-human primates (NHPs) used for this type of pre-clinical modeling, the team at Tune had to use a proxy target: PCSK9, a liver enzyme that controls levels of low-density lipoprotein C (LDL-C) and contributes to cardiovascular disease.
If this gene sounds familiar, it’s because it’s currently being targeted in a CRISPR-Cas9 trial sponsored by Verve Therapeutics to treat high-risk heterozygous familial hypercholesterolemia and established atherosclerotic cardiovascular disease.
»What we've actually done is relied on PCSK9 gene repression as a model system for HBV. The idea is that we could use the same epigenetic editor protein and the same LNP, delivered to liver cells. The regulation of PCSK9 is very similar to HBV - there are regions that can be epigenetically repressed to control transcription,« Cosgrove explained.
Using this proxy model, Tune have shown that a single dose of TUNE-101 is well-tolerated by the animals, achieving a 75% reduction in PCSK9 protein in serum and a resulting 56% reduction in LDL-C levels (see Figure 2 below). These responses seem to be very durable; the reduction in PCSK9 and LDL-C is still holding strong past 1 year, and the therapeutic methylation patterns are primarily unchanged over the same period.
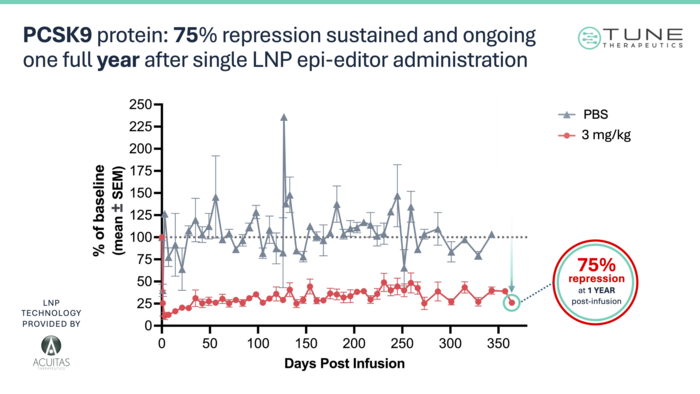
»Right now, we're pretty far along in our pre-clinical package. The goal is to be able to complete a clinical trial agreement and dose our first patient by the end of this year,« Cosgrove commented.
Assuming the therapeutic strategy is successful in human trials, Cosgrove and the team at Tune are optimistic about the future rollout of TUNE-401, even in lower and middle-income countries where HBV is most prevalent.
»This is an mRNA-based, LNP delivered therapy,« said Cosgrove. »You could take a therapy like that, and roll it out to the entire world, as we’ve seen with COVID, and produce this at scale and drive down costs. It’s something that isn't quite there yet… But I'm really hopeful about how we scale out mRNA based therapies, and I think that these can truly be accessible to other parts of the world.«
Relevant links
- Tune Therapeutics Reveals Epigenetic Editing Program Targeting Hepatitis B Virus. Press release by Tune Therapeutics unveiling the TUNE-401 programme (November 2023).
- Overview of Tune Therapeutics' TEMPO platform.
Rebecca Roberts is a molecular biologist, science writer/communicator, and scientific marketing professional based in Queensland, Australia.
This article was originally published on 4th March 2024. It was updated by CMN on 11th March 2024. During this update, Figure 2 was replaced with a newer figure to include post-editing data over a longer period (greater than 1 year versus 280 days in the original Figure 2). In addition, the therapeutic target of TUNE-401 has been reformulated to 'master controller sequence' as opposed to 'master controller gene'.
To get more CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
ArticleInterviewNewsHepatitis B virusViral diseaseEpigenome editing (e-GE)Tune Therapeutics
CLINICAL TRIALS
Sponsors:
Wave Life Sciences Ltd.







