Your missing links are here (31 July 2020)
By: Rasmus Kragh Jakobsen - Jul. 31, 2020
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...
Top picks
- Researchers have invented a genetic safety mechanism that can deactivate transplanted cells if they change in a problematic way. Wiebking et al. used gene-editing to engineer the metabolism of human cells to render them auxotrophic.
- Megaphages harbour mini-Cas proteins ideal for gene-editing. Small Cas for big use. And also: Tiny Cas Protein, Huge Gene Editing Potential … Thanks, Megaphage!
- Amazing set of stories from the NYTimes on families confronted by rare genetic diseases and their approaches to find therapies. We got goosebumps thinking about the struggles and how those families found the energy to keep fighting.
Research
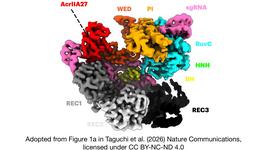
- The structure of a CRISPR-Cas9 base editor (ABE8e) is revealed for the first time in ScienceMag. Our story on capturing the base-editor in the act using cryo-EM!
- More atomic details! Impressive cryo-EM structure of the largest CRISPR-Cas effector complex in Molecular Cell. Here is the story and a review on the structural basis of CRISPR-Cas Type III in Current Opinion in Structural Biology.
- New CRISPR C-to-G DNA base editor expands the landscape of precision genome editing. Nature study. Another take on the story here.
- Base-editing reveals gene behind eye health in cavefish. The same gene is responsible for homocystinuria in humans, and the study may provide clues about human eye diseases.
- Prediction-based highly sensitive CRISPR off-target validation using target-specific DNA enrichment.
- Expanded ENCODE delivers invaluable genomic encyclopedia.
Industry
- Olympus and Cytosurge collaborate to deliver a complete single-cell and CRISPR genetic manipulation solution in the Americas.
- New startup to develop novel delivery methods for gene-editing and other cargos.
- Editas Medicineenter partnership for manufacture and supply of gene editing portfolio.
- CARISMA Therapeutics Announces FDA Clearance of IND Application for First-Ever Engineered Macrophage Immunotherapy.
COVID-19
- The explosion of new coronavirus tests that could help to end the pandemic. Research groups around the world are devising diagnostic tests that go beyond the gold standard PCR, and churn out millions of tests a week. Some use CRISPR gene-editing to home in on genetic snippets of SARS-CoV-2.
- Promising results in BioRxiv preprint. Rapid, sensitive and specific SARS coronavirus-2 detection: a multi-center comparison between standard qRT-PCR and CRISPR-based DETECTR.
- Anthony Fauci interview by Ed Yong on The Atlantic.
- George Church and other scientist are sniffing home-made COVID19 vaccines.
Vision and opinion
Policy
- UK anti-GMO groups aim to block relaxed CRISPR crop rules gaining bipartisan support in Parliament.
Heh, huh, wow
- The making of a scientist. Nobel Laureate Mario Capecchi’s story is a story of hope for those who struggle early in life. »… at age 4½, I set off on my own. I headed south, sometimes living in the streets, sometimes joining gangs of other homeless children, sometimes living in orphanages, and most of the time being hungry...« Mario Capecchi, born 1937, spent years on the streets in Italy and nearly died of malnutrition in a hospital. In 2007, he was awarded the Medicine Prize »for their discoveries of principles for introducing specific gene modifications in mice by the use of embryonic stem cells«.
- Say moo to Cosmo the CRISPR calf, and the many CRISPR challenges the scientists ran into. UCDavis also has the story.
- Lab-grown sperm could let infertile men have gene-edited children.
Tags
CLINICAL TRIALS
IND Enabling
Phase I
Phase II
Phase III
Gastric Cancer and Colorectal Cancer, CRC, (NCT07166263)
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
IND Enabling
Phase I
Phase II
Phase III
Relapsed or Refractory Acute Myeloid Leukemia, AML, (NCT06541444)
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
IND Enabling
Phase I
Phase II
Phase III