AIRNA: Harnessing the Body’s RNA-Editing System for Therapeutic Applications
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...

RNA editing has emerged as a promising new approach in genetic medicine, offering potential advantages over DNA editing in treating human diseases. AIRNA, a Boston- and Germany-based biotechnology company is advancing a therapeutic platform based on ADAR (adenosine deaminase acting on RNA), an RNA-editing enzyme endogenous to human cells.
The company, co-founded by Thorsten Stafforst at University Hospital Tübingen in Germany, Jin Billy Li at Stanford University, VP of Research Paul Vogel and VP of Platform Tobias Merkle, builds upon more than a decade of pioneering research in ADAR biology and RNA-editing technologies.
Despite debuting as recently as September 2023, AIRNA is already preparing to bring its first candidate, targeting alpha-1 antitrypsin deficiency (AATD), into clinical trials later this year. This rapid progress reflects the company's broader vision for RNA-editing therapeutics to treat not only the most vulnerable, but also the most people living with rare and chronic genetic diseases.
»The profile of a medicine required to treat large populations is quite different to a therapy designed to treat a small group of severe patients,« notes AIRNA's President and CEO Kris Elverum. »Many people that have a genetic disease are not facing a short life expectancy and want a medicine that allows them to live healthy lives without a significant risk of side effects.«
CRISPR Medicine News spoke with Thorsten Stafforst and Kris Elverum to learn more about ADAR, the company’s lead programme in AATD and visions for the future.
Meet Thorsten Stafforst at the 2nd CRISPR Medicine Conference, CRISPRMED25, in Copenhagen, Denmark, April 8-11, 2025.
ADAR: Nature's RNA editor and its critical role in immune defence
»ADAR is an enzyme which is found in almost all cells in the body and this ubiquitous presence serves an essential function,« explains Stafforst, »ADAR is a nuclear protein that acts as an immune suppressor by editing the cell's own double-stranded RNA (dsRNA) in the nucleus and the cytoplasm, preventing it from triggering immune responses typically reserved for viral RNA.«
Whereas viral dsRNA is recognised by specialised sensors, most notably MDA5 and RIG-I, our own dsRNA is edited by ADAR so that it will not be recognised by those receptors.
The human genome encodes multiple ADAR proteins, with ADAR1 and ADAR2 being the primary editing enzymes. While ADAR1 mainly serves the immune regulatory function described above, its close homologue ADAR2 performs specific editing events, such as modifying single amino acids in neurotransmitter channels to modulate their function, e.g., in glutamate and serotonin receptors.
Inside ADAR's editing process: Programmable, precise, and efficient
ADAR enzymes perform precise chemical modifications, converting adenosine (A) to inosine (I) in RNA molecules. According to Stafforst, the classical substrates for ADAR are Alu repeats, which are the most abundant type of repetitive element in primate genomes.
Because of their repetitive nature, transcribed Alu repeats readily form a looped dsRNA structure, which activates dsRNA sensors to trigger a strong innate immune response if not removed. ADAR is recruited specifically to those dsRNA structures, and this forms the basis for their programmability for therapeutic applications, as explained by Stafforst:
»The beauty of ADAR is its double-strand specificity, which we can harness to target any adenosine in the transcriptome. If the target adenosine is single-stranded, which is most often the case, we can create an oligonucleotide or guide RNA that goes to the nucleus and base-pairs with the target to form a suitable dsRNA substrate for ADAR.«
Simple as it sounds though, defining a ’good’ ADAR substrate has not been straightforward but it is key to the success of AIRNA’s approach. »We have spent a lot of time developing sophisticated chemistry to optimise and modify the guide molecules, making them stable enough for therapeutic benefit while ensuring they direct editing to precise locations within target RNAs. These modifications include changes to the ribose backbone and specific chemical alterations that enhance stability while maintaining ADAR recognition. With those modifications, we can narrow down the editing window to one nucleotide and virtually achieve bystander-free editing, as we have shown in our AATD programme,« said Stafforst.
ADAR editing: Simplicity meets therapeutic versatility
Of note, ADAR is distinct from CRISPR-based editing approaches in that it neither requires nor acts against any DNA repair pathway: »The A-to-I edit induced by ADAR is remarkably efficient because it doesn't require DNA repair pathways. It's really just a flip out hydrolysis reaction at the base. You do not cut any backbone and the edit is precise. The target adenosine is flipped out, edited in the active site and flipped back in,« Stafforst explains.
The only reagent required for ADAR editing is the target-specific guide RNA or antisense oligonucleotide and a delivery vehicle. The company has tested many primary cell types and immortalised cell lines and the amount of endogenous ADAR was never limiting. The short length of the oligonucleotides results in a very small landing platform for ADAR, which further strengthens the specificity of the approach.
While AAV-based delivery of guide RNAs may be an easier way to achieve proof-of-concept in animals, it is not Stafforst’s preferred delivery route: »I personally don't believe this is the sweet spot of our technology because it doesn’t make use of the transient nature of editing. A permanent guide RNA that is permanently expressed from an AAV will lead to permanent correction and that may be good for correcting genetic disease at the DNA level. But at AIRNA, we are developing chemically-defined RNA-editing medicines that are like traditional medicines. This also puts us on a different road to address gain-of-function targets and large diseases with high unmet need, unlike other gene editing approaches.«
While ADAR-based editing is not mutation-agnostic, AIRNA has demonstrated its suitability for multiplexing and it can reverse G-to-A mutations – which are frequently encountered. In principle, up to 12 amino acids, the START and all three STOP codons can be manipulated resulting in up to 16 different amino acid substitutions, which is expected to cover a broad landscape of monogenetic disorders. Even when reversal of a mutation to wild-type is not possible, it may be sufficient to engineer the target mRNA transcript to produce a protein with enough functionality for therapeutic benefit. Furthermore, splice elements could be weakened or strengthened by ADAR-induced editing.
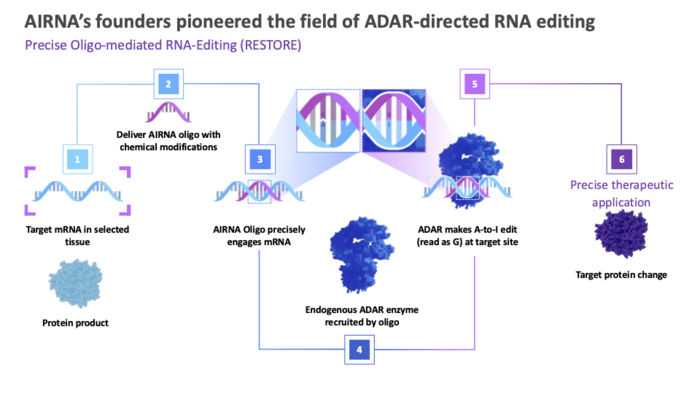
So, why is RNA editing preferable to permanent DNA correction?
While DNA editing approaches such as CRISPR-Cas and base editing are proving successful in treating severe genetic diseases, Elverum points out that RNA editing offers distinct advantages for broader therapeutic applications. According to him, the transient nature of RNA editing, often seen as a limitation, becomes an advantage in this context. It allows for dose adjustment or discontinuation if needed, providing patients with greater control over their treatment.
»This approach could enable earlier intervention in disease progression, as patients and physicians might be more comfortable trying a reversible therapy. Most people want to have control over their health throughout their lives,« said Elverum, who elaborates:
»I think the field of RNA editing has the opportunity to fill that gap. We're now able to make individual letter changes, but with a drug product that is temporary. You can dose up or down, start or stop, while keeping it accessible through subcutaneous, infrequent administration and scalable manufacturing. As a result, from an access perspective, RNA editing is fundamentally different than other gene therapies.«
Another significant advantage of RNA editing lies in its specificity. According to Elverum, particularly in the case of the SERPINA1 gene targeted in the company’s AATD programme, RNA editing has shown superior specificity compared to DNA base editors.
AATD: A strategic first programme
AIRNA's lead programme targets AATD, a genetic disorder primarily affecting the lungs and liver, and which is estimated to affect 100,000 people in the United States alone. The disease is largely caused by a specific mutation (PIZZ) in the SERPINA1 gene, which results in the production of a defective protein in the liver. This mutation leads to both a loss of functional alpha-1 antitrypsin protein in the blood and accumulation of misfolded protein in liver cells, potentially causing lung and liver disease. Current treatment strategies focus on symptom management and typically involve the use of bronchodilators, antibiotics and, in some cases, injections of replacement AAT protein.
The choice of AATD as a first target was strategic, allowing the company to prove its technology while minimising risks. »AATD is a very uniquely fit for purpose disease for our technology,” explains Elverum. »We understand the biology and the genomics that are causing the disease. We also know what cells are affected and we have the means to deliver our therapy to those cells.«
Delivery innovation
AIRNA's delivery approach represents a significant advancement in the field of oligonucleotide therapeutics. The company has opted for direct conjugation of their antisense oligonucleotide molecules to GalNAc, avoiding the use of lipid nanoparticles (LNPs), which are used in Beam Therapeutics’ ongoing trial of base-editing candidate BEAM-302 in AATD and Korro Bio’s recently approved trial of ADAR candidate KRRO-110 in AATD.
GalNAc conjugation takes advantage of the asialoglycoprotein receptor (ASGPR) which is highly expressed on hepatocytes, enabling very specific delivery to liver cells. This strategy builds on successful precedents in the siRNA field, where GalNAc-conjugated molecules have shown superior properties compared to LNP-delivered drugs.
Importantly, this delivery method may bypass the need for the steroid pre-treatments given ahead of LNP-delivered drugs. »The use of LNPs is associated with toxicity, including inflammatory effects which have been dose-limiting. In contrast, GalNAc-mediated delivery has demonstrated a better safety profile and enables higher dosing levels. This approach also appears to provide more sustained therapeutic effects, potentially allowing for less frequent dosing schedules,« explains Elverum.
Within this emerging field of ADAR editing for AATD, AIRNA isn't alone in pursuing GalNAc-mediated delivery. Wave Life Sciences is also developing a similar therapeutic approach using this delivery method. Both companies have made modifications to the standard GalNAc conjugation techniques traditionally used for siRNA or ASOs to better suit ADAR-directed RNA editing. The optimisation of these delivery systems represents an important area of development in making RNA-editing therapies both effective and convenient for patients.
AIRNA expects to file for clinical trial authorisation in 2025, with the goal of demonstrating a best-in-class medicine combining optimal potency with convenient administration. »Based on our data, and across multiple models and multiple species, we're really encouraged by the target product profile that we're developing,« Elverum notes.
Looking beyond rare disease
While AIRNA's initial focus is on correcting disease-causing mutations in mRNA, e.g., in AATD, the company also sees broad potential in modulating wild-type proteins for therapeutic benefit in common diseases. »We think what's more interesting and aligns with the long-term promise of ADAR editing is to target wild-type alleles. This could include introducing protective variants or modifying receptors or altering protein function, e.g., through targeted editing of sites involved in post-translational modifications,« said Stafforst.
Stafforst's academic research team at University Hospital Tübingen is now developing a discovery platform to identify interesting new targets for ADAR to modify protein post-translational sites or alter protein-protein interactions, potentially opening new therapeutic possibilities. Meanwhile, AIRNA is exploring opportunities in cardiometabolic diseases and other therapeutic areas, though specific programmes beyond AATD remain undisclosed.
Elverum also has a clear vision of RNA editing beyond the treatment of rare diseases:
»It's not just about identifying and targeting mutations that cause severe disease, but also finding protective variants,« Elverum explains. »For example, take a 90-year-old smoker who doesn't have heart disease. Could we identify a protective variant in this person, and then introduce that protective variant to a larger population? This approach opens a wide range of possibilities that could help broad patient populations.«
Reflecting on the expansion of the gene-editing toolbox as new technologies emerge and advance, Elverum said: »I think there's opportunity for everybody to be successful in this field. RNA editing can serve such a different purpose than other gene-editing modalities. We have the ability to fine tune and make just a single individual letter change in a way that can be profoundly beneficial to the patient.«
To get more CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
ArticleInterviewNewsAlpha-1 Antitrypsin Deficiency, AATDRNA editingADAR (adenosine deaminase acting on RNA)AIRNA
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.







