An Innovative Approach to Treating Cystic Fibrosis Using Base Editors
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...

It might seem counter-intuitive that one mutation could alleviate the effects of another, but this might be the key to finally developing a successful CRISPR-based therapy for cystic fibrosis (CF). In a study published recently in Molecular Therapy, researchers from the University of Trento, Italy, used a base-editing system to insert revertant mutations in the CFTR gene that compensate for a disease-causing mutation. The proof-of-concept study used patient-derived primary epithelial cells supplied by the Italian Cystic Fibrosis Research Foundation, who also provided funding for the research.
»In the beginning, I didn’t really believe that this was possible«, said Giulia Maule, a postdoctoral researcher in Anna Cereseto’s laboratory at the University of Trento and co-author of the paper. »I thought, come on, it's impossible that one mutation can compensate for another one. So, when we saw that this was actually the reality, it was kind of a wow moment - it is possible.«
Cystic fibrosis and CFTR mutations
CF is caused by multiple autosomal recessive mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. Encoding a chloride ion channel in the membrane of epithelial cells, CFTR regulates the flow of chloride ions in and out of these cells.
CFTR mutations result in insufficient production of the ion channel or impair its function; the mucus layer on the surface of airway epithelial cells becomes dehydrated and sticky and accumulates in the airways. Frequent respiratory infections are common and can be fatal, with mortalities most often caused by obstructive lung disease.
While more than 700 known CFTR mutations are associated with CF, the F508del mutation is by far the most common, accounting for 80% of cases. A deletion of three nucleotides results in the loss of a phenylalanine residue in the protein, causing it to misfold. The misfolded protein is subsequently targeted for degradation via the ubiquitin-proteasome system. There is no known cure for CF; small-molecule drugs that modulate CFTR protein folding or gating activity are the only available treatments.
Multiple attempts to develop gene therapy for cystic fibrosis
Before CRISPR, several unsuccessful clinical trials attempted to use gene replacement therapy to treat CF. Several proof-of-concept studies have followed using CRISPR-Cas9 to correct the mutations in CFTR, yet several key obstacles remain. Correcting the mutations in vivo requires a Cas9 nuclease, a guide RNA, and a template for homology-directed repair (HDR). The protein-coding region of the CFTR gene alone is 4.5 kilobases (kb), making it difficult to deliver using viral vectors such as adeno-associated viruses (AAV), which have a packaging limit of 4.7 kb.
Lipid nanoparticles (LNPs) lack the packaging constraints of AAVs but have only recently been adapted to target the lungs. Additionally, the lungs have a low rate of cell turnover, making it extremely difficult to perform efficient in vivo gene editing. While recent studies have shown that CFTR mutations can be corrected in patient-derived basal airway stem cells and human bronchial epithelial cells ex vivo, the transplantation and engraftment of these edited cell therapies in the airways present more challenges.
Moreover, gene -editing technologies such as CRISPR-Cas9 that induce double-stranded breaks (DSBs) in the genome carry the risk of off-target editing and other unintended consequences. This was an important consideration for Maule, who has worked in CF research since her PhD. »Up to that point, we used the Cas9 as a nuclease, and we knew that it was introducing uncontrolled modifications into the genomic target site, even though it proved to be useful [in correcting the mutations],« she said.
The team decided to find a more precise and controlled method, and the subsequent development of base editors (BEs) provided the answer. The use of BEs circumvents two key obstacles; base editors (BEs) do not require a template for HDR, making the cargo much smaller for delivery, and they do not induce DSBs in the genome.
»The strategy to introduce revertant mutations in the CFTR gene through base editing is innovative,« remarked Dr. Shafagh Waters, who leads a cystic fibrosis research programme at the University of New South Wales and was not involved in the study. »This method could be safer than traditional gene-editing approaches like CRISPR-Cas9.«
Revertant mutations and CFTR rescue
Rather than trying to correct the F508del mutation, Maule and her colleagues explored the possibility of using BEs to introduce revertant mutations (RMs): naturally occurring mutations in CFTR that can compensate for the presence of the pathogenic deletion. First discovered in the early 90s, RMs can restore the correct folding and function of the CFTR protein.
»There were patients that had the F508del mutation, but they were also experiencing a milder phenotype compared to other patients. By sequencing, [researchers] found these revertant mutations,« explained Maule.
This approach may sound familiar to those who have followed the development of CRISPR-based therapies for sickle cell disease (SCD). One of the more unorthodox attempts involved using base editors to replace the disease-causing GTG mutation in the haemoglobin gene with a naturally-occurring, non-pathogenic GCG mutation known as the Makassar variant, restoring the production of haemoglobin.
Depending on each RM, Maule and the team used either adenine base editors (ABEs) or cytosine base editors (CBEs), testing several different SpCas9 variants and sgRNAs for each to identify the best combination. They investigated which of several RMs were able to produce a mature CFTR protein in vitro in immortalised HEK293 cells, then explored its cellular localisation to ensure it was trafficked to the cell membrane.
After completing functional assays, they selected several RM candidates for further testing. The group also explored the possibility of introducing combinations of RMs to the cells, in the hope of increasing functional rescue.
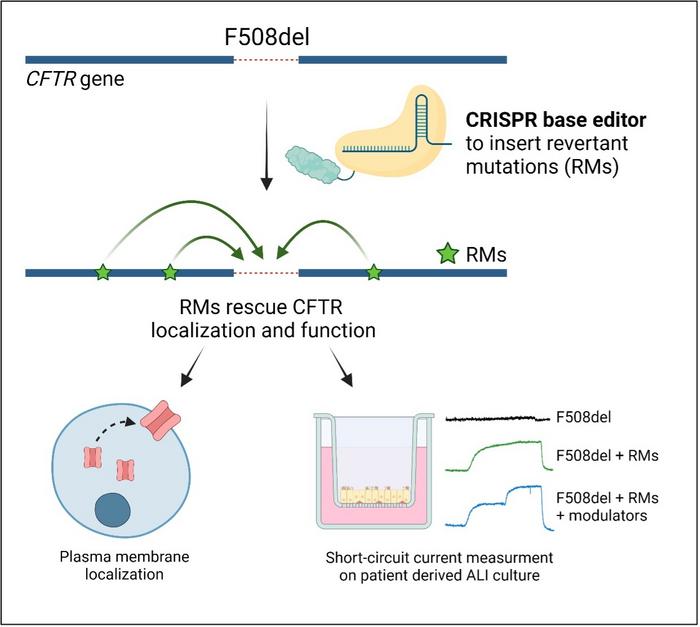
Next, Giulia and the team transferred the approach into a CF model: primary bronchial epithelial cells (HBE) derived from F508del homozygous patients. »The most challenging part is to move the editing efficiencies that you achieve in the immortalised cell lines into primary cells,« commented Maule.
Luckily, they were able to achieve editing efficiencies very similar to those seen in the HEK293 cells. Delivering the BE mRNA and sgRNA to the cells via electroporation, they introduced the R555K and R1070W RMs to the HBE cells. Whole-genome off-target editing analysis and deep sequencing of potential off-target sites demonstrated minimal off-target activity.
To further characterise the functional rescue of CFTR, they used the edited HBE cells to create terminally differentiated, pseudostratified epithelia, which they cultured under air-liquid interface conditions to mimic the in vivo environment of human airways. While no significant improvement was observed in epithelia carrying the R555K RM, those carrying the R1070W RM showed a 15-20% activity rescue, while the combination of R555K + R1070W yielded 23-31% rescue.
Maule and her colleagues also tested currently available CFTR-modulating drugs in the edited cells. When treated with ivacaftor, a potentiator that improves CFTR gating, cells edited with R1070W and R555K + R1070W RMs showed further increases in functional activity.
Waters said this approach could potentially be applied to a large number of CF patients. »The potential to combine genetic correction with pharmacological modulators to treat cystic fibrosis could set a new standard in therapeutic strategies,« Waters remarked.
Maule explained that despite some challenges, this approach is most likely to be adapted as an in vivo gene therapy. »We are working on using virus-like particles (VLPs) to achieve transient delivery of the base editor,« she said. »This will allow us, in theory, to reach the lung, correct the cells, and then have the CRISPR tool degraded quite fast…to avoid long-term expression.«
Another important next step for Maule and her colleagues is to test their approach in animal models. »Animal studies are crucial at this stage to confirm the approach's efficacy, delivery and safety. These studies would help validate the delivery method and the long-term stability of the edited genes in the lung microenvironment,« said Waters.
This presents a challenge for the team, since there is a paucity of appropriate animal models of CF. Mice, for example, are not an accurate model because they do not develop CF disease phenotypes in their lungs. Maule and her colleagues continue to work toward studies in larger animal models, such as pigs.
»I am eager to see how further research will build on these promising results, especially through well-designed animal studies and eventual clinical trials,« Waters concluded.
Rebecca Roberts is a molecular biologist, science writer/communicator, and scientific marketing professional based in Queensland, Australia.
Link to the original article in Molecular Therapy:
Functional rescue of F508del-CFTR through revertant mutations introduced by CRISPR base editing.
To get more CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.







