BEAM-101: IND Approval for First Ever Base-Edited Therapy
BEAM-101 is a patient-specific, autologous haematopoietic cell therapy designed as a one-time treatment for sickle cell disease (SCD) and beta-thalassemia (BT). These diseases belong to a larger family of diseases known as the haemoglobinopathies, which are caused by a lack of functional adult haemoglobin. SCD results from a single-point mutation in the haemoglobin subunit beta (HBB) gene, while BT may arise from one of >200 known HBB mutations.
Foetal haemoglobin (HbF) is highly expressed and critical during foetal development, but then rapidly suppressed early in life, when its role is taken over by what we know as adult haemoglobin. BEAM-101 cells incorporate ex vivo base edits that mimic single nucleotide polymorphisms seen in individuals with hereditary persistence of HbF.
The therapeutic strategy behind BEAM-101 is that reactivation of HbF expression will compensate for the lack of functional adult haemoglobin seen in SCD and BT. Although BEAM-101 is designed to treat both SCD and BT, the recent IND approval only pertains to SCD.
Precise single base changes without double-strand DNA breaks
First developed in 2016 in the lab of Dr. David Liu – who is also a co-founder of Beam Therapeutics, base editors are derived from CRISPR-Cas technology as engineered fusion enzymes that can specifically modify a single cytidine (cytosine base editor, CBE) or adenosine (adenine base editor, ABE) base in genomic DNA.
One of the most attractive features of base editors is their ability to make precise single-base changes in DNA without introducing a double-strand break, raising major hopes that they will offer safe correction of genetic diseases that are caused by a single-point mutation.
Findings from studies in animal models have provided compelling evidence for the ability of base editors to correct several rare genetic diseases including progeria, Leber congenital amaurosis, SCD, and Duchenne muscular dystrophy, and the recent IND approval for BEAM-101 marks the very first base editor to edge towards the clinic.
BEAM-101 reactivates foetal haemoglobin
Decades of research to decipher the genetic pathways involved in regulating adult and foetal haemoglobin production have opened doors for gene-editing approaches that aim to treat or even cure the haemoglobinopathies, many of which involve reactivation of HbF.
BEAM-101 is the first base-edited therapy candidate for a haemoglobinopathy. BEAM-101 cells are engineered ex vivo with an ABE that incorporates A → G base edits in the HBG1 and HBG2 gene promoters, which regulate the expression of HbF. These edits mimic the naturally-occurring HbF-inducing mutations observed in individuals that continue to produce foetal haemoglobin beyond infancy, leading to reactivation of HbF expression to compensate for the lack of adult haemoglobin in SCD.
The BEAM-101 base-editing reagents are delivered to patient-derived haemopoetitic stem cells via electroporation. Results from animal studies reveal high levels of base editing and robust HbF induction after long-term in vivo engraftment with BEAM-101 cells (Figure 1).
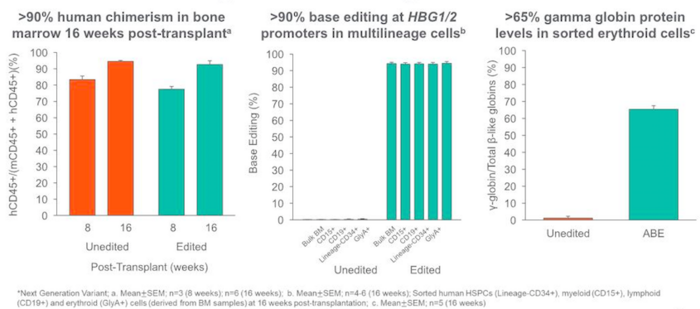
BEACON-101 trial
According to a company press release, BEAM-101 is envisioned to be a one-time treatment for patients with SCD, and plans are underway to initiate the BEACON-101 trial, which will be a Phase 1/2 clinical trial to assess the safety and efficacy of BEAM-101 for the treatment of SCD.
We will continue to provide updates about BEAM-101 and the BEACON-101 trial as further details emerge.
For a complete overview of CRISPR IND approvals and ongoing gene-editing clinical trials, check out CRISPR Medicine News' Clinical Trials Database.
Related CMN articles:
Toward Safer Genetic Engineering, One Base at a Time (An explainer about base editing)
To get more of the CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
ArticleMost readNewsin vivoElectroporationBeta ThalassemiaSickle Cell Disease, SCDBlood diseaseRare DiseaseBase editorsBeam Therapeutics Inc.TrialsIND - Investigational New Drug
CLINICAL TRIALS
Sponsors:
Wave Life Sciences Ltd.







