Breaking: First personalised mRNA CRISPR therapy delivered in six months
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...
The study represents a proof of concept for bespoke gene editing as a clinical strategy, offering a potential model for future treatment of rare genetic conditions. The patient, a neonate with two truncating CPS1 mutations, presented with hyperammonaemia and was at risk of irreversible neurological damage or death.
Conventional treatment, including protein restriction and nitrogen scavengers, proved insufficient. A liver transplant was being considered, but the clinical team pursued an experimental alternative: a fully personalised CRISPR base-editing therapy delivered systemically using lipid nanoparticles.
“What we’ve accomplished together sets a new gold standard for operationalising the future of medicine”Sandy Ottensmann, IDT
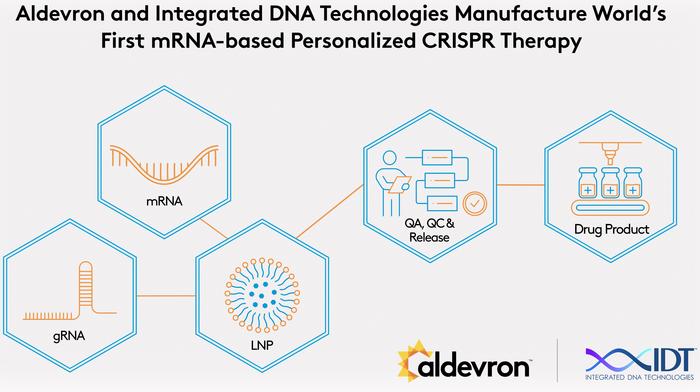
The therapy – named “k-abe” – combined a newly designed guide RNA (“kayjayguran”) targeting the specific Q335X mutation and a custom mRNA encoding an adenine base editor (NGC-ABE8e-V106W). The editor was formulated and manufactured through collaboration with Aldevron and Integrated DNA Technologies (IDT), both subsidiaries of Danaher Corporation.
Notably, the development pipeline – from genetic diagnosis to clinical-grade drug product – was compressed to just half a year, approximately three times faster than standard timelines.
»We are unique in our ability to deliver this innovative treatment in such a short timeline,« said Mark Wetzel, VP of mRNA CDMO Services at Aldevron. »This CRISPR therapy was made under exceptional circumstances […] to do what was needed to improve this patient’s outcome.«
Following two intravenous infusions at 7 and 8 months of age, the patient showed reduced blood ammonia concentrations, improved tolerance to dietary protein, and sustained clinical stability despite concurrent viral infections. No serious adverse events were reported.
Synonymous bystander editing was observed, and a low level of off-target editing was detected at a single intronic site in ATP7B in vitro but deemed clinically irrelevant. Liver biopsy was avoided due to risk, so direct confirmation of in vivo editing in the patient is not yet available.
The rapid design and deployment of this “N-of-1” therapy relied on an extensive preclinical evaluation of editing specificity and efficiency using in vitro models and mouse systems. The same sequence cassette harbouring the CPS1 Q335X variant was used across in vitro screens, murine liver models, and cynomolgus monkey safety assessments.
“This work suggests a potential roadmap for transforming CRISPR therapies for other inborn errors of metabolism and life-threatening genetic diseases”Kiran Musunuru, University of Pennsylvania
The favourable safety profile supported regulatory authorisation for a compassionate-use protocol. Off-target analyses using CHANGE-seq and ONE-seq, tailored to the patient’s genome, enabled confidence in therapeutic selectivity.
Sandy Ottensmann, VP of Gene Writing & Editing at IDT, underscored the significance of the effort: »What we’ve accomplished together sets a new gold standard for operationalising the future of medicine. The implications of this work are profound and illuminate how collaborations between academic medicine and industry can enable major science wins.«
The personalised therapy was manufactured through collaboration between academic researchers and several industry partners. Aldevron produced the mRNA component; IDT supplied the guide RNA and conducted off-target analyses; Acuitas Therapeutics provided the clinically validated lipid nanoparticle formulation.
The platform nature of the components – where only the guide RNA needs to be tailored – enabled rapid modular development. The success of this approach aligns with the mission of the Danaher–Innovative Genomics Institute Beacon for CRISPR Cures, launched in 2024, to accelerate patient-specific genome editing.
»The impact of this work extends beyond this particular patient and category of clinical indications,« said senior author Kiran Musunuru, MD, PhD, of the University of Pennsylvania. »It suggests a potential roadmap for transforming CRISPR therapies for other inborn errors of metabolism and life-threatening genetic diseases.«
To get more CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
ArticleInterviewNewsin vivoLipid-based nanoparticleMetabolic disordersBase editors
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.








