Breaking: The 2020 Nobel Prize in Chemistry goes to Emmanuelle Charpentier and Jennifer Doudna for developing CRISPR
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...
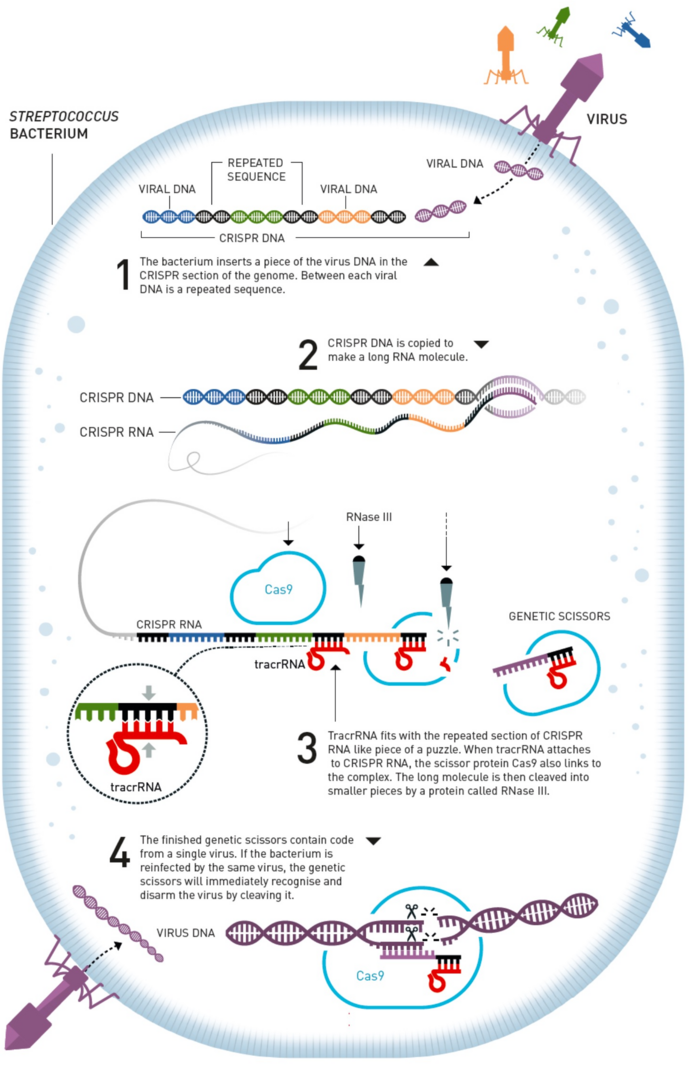
The history of CRISPR began millions of years ago when bacteria desperately needed to defend themselves against almost constant attacks by virus-like bacteriophages, or just phages, that outnumber bacteria by a factor of ten in most ecosystems.
Bacteria developed a defence mechanism that in many ways resembles our acquired immune system. They learned to copy small pieces of the phage DNA to keep a blueprint of the phage in memory and also developed a genetic scissors that was able to cut these genetic sequences in two in a sequence-dependant manner.
Both parts of this machinery are encoded by bacterial genes that have spread through the bacterial kingdom such that most if not all bacteria (and archaea) now possess this defence system. Simply put, when bacteria encounter a phage, they snap up a piece of the phage DNA and use it to target their genetic scissors to the phage genome and cut it up.
In 2007, researchers Philippe Horvath and Rodolphe Barrangou from Danisco - a food ingredients company with headquarters in Denmark - were the first to understand that bacteria used CRISPR as a defence mechanism against phages. They soon they found ways to exploit the system in the dairy industry where phage infections by fermenting bacteria can lead to spoilage of cheese and yoghurt.
The Revolution Begins
At that time, nobody knew how the bacterial defence system worked, and so the world was unaware of its huge potential. However, that changed when Emmanuelle Charpentier and Jennifer Doudna met in 2011 and started to unravel the underlying mechanisms. Emmanuelle Charpentier was then at Umeå University in Sweden and Jennifer Doudna at the University of California, Berkeley in the USA.
The two researchers started to elucidate the components of CRISPR - Cas9, gRNA, etc. - and they showed that any sequence could be targeted for cutting. The discovery sparked immense interest with numerous other researchers, e.g., Feng Zhang from Massachusetts Institute of Technology (Boston, USA), rapidly joining the new scientific field, and things really started to go fast.
Quickly, CRISPR-Cas9 became a simple, cheap and widespread tool to knock out genes and introduce mutations or deletions with unprecedented precision that left many previously used genetic engineering tools behind. Already in 2014, American researchers used CRISPR to correct a mutation in the Fah-gene in mice and partially cure them of the human disease hereditary tyrosinemia.
Since then numerous other genetic diseases have been targeted and crops have been edited for better quality or pest resistance. Moreover, the basic CRISPR technology has been expanded with new Cas endonucleases as well as new concepts such as prime editing, base editing and epigenetic editing, to name a few.
By awarding Emmanuelle Charpentier and Jennifer Doudna the Nobel Prize just nine years after they uncovered the basics of CRISPR, the Royal Swedish Academy of Sciences emphasises that CRISPR-Cas9 and all its facets have started a real revolution in molecular biology.
Tags
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.







