Clinical Update: CRISPR-Edited Stem Cell Therapy for Acute Myeloid Leukaemia
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...
Vor Biopharma, headquartered in Massachusetts, is developing VOR33 as an allogeneic cell therapy product to replace the current standard of care in allogeneic haematopoietic cell transplant (HCT) settings for patients with AML who are at high risk of relapse.
Allogeneic haematopoietic cell transplant for AML
Human leukocyte antigen (HLA)-matched allogeneic HCT is widely used in the long-term management of AML following prior treatment with chemotherapy and/or radiation.
Although HCT is generally seen as curative option for AML, approximately 40% of all AML patients relapse despite HCT from a matched donor. Post-HCT targeted chemotherapy to reduce relapse in these high-risk patients is limited by toxicity to healthy patient cells as well as on-target toxicity to newly engrafted cells.
CRISPR-Cas9 disruption of CD33 for durable anti-tumour therapy
CD33 is a cell surface antigen that is abundantly expressed in the vast majority (80%) of AML cases, and it is one of the main validated target antigens in AML so far.
Several programmes are ongoing to target CD33 in AML, but so far gemtuzumab ozogamicin (Mylotarg™) is the only CD33-directed antibody-drug conjugate to be approved by the FDA and European Medicines Agency (EMA) for the treatment of AML.
VOR33 is Vor Biopharma’s lead candidate and is developed from healthy donor haematopoietic stem and progenitor cells (HSPCs). These cells are subjected to CRISPR-Cas9 genome-editing to disrupt the expression of the CD33 protein. The company’s goal is to advance VOR33 as a new standard treatment for high-risk CD33+ AML. They plan to administer the anti-CD33 antibody Mylotarg™ after HCT to target residual CD33+ AML cells without toxicity to the engrafted VOR33 cells.
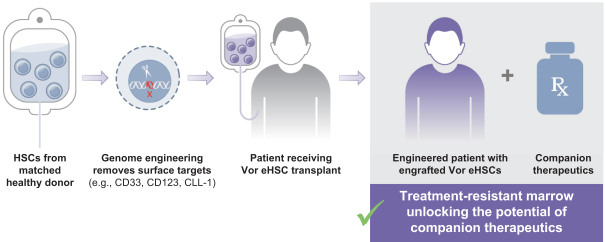
VBP101 trial for VOR33 in high-risk AML
In a Phase 1/2a, multicenter, open-label, first-in-human (FIH) study, Vor Biopharma will assess the safety and efficacy of VOR33 in participants with AML who are currently undergoing donor-matched HCT. The study is scheduled to begin this year and will enrol 18 adult participants (18-70 years of age) who will be split into 3 experimental cohorts.
All participants will undergo a myeloablative HCT with matched related or unrelated donor HSPCs engineered to remove CD33 expression (i.e. the VOR33 product). Mylotarg™ at one of three different doses (one for each experimental cohort) will be administered after VOR33 engraftment for up to 4 cycles.
The primary endpoint assessing safety of VOR33 will be the incidence of successful VOR33 engraftment at 28 days. Part 1 of this study will evaluate the safety of escalating Mylotarg™ dose levels to determine the maximum tolerated dose (MTD) and recommended Phase 2 dose (RP2D). Part 2 will expand the number of participants to evaluate the Mylotarg™ RP2D.
The estimated primary completion date, i.e., the date on which the last participant is examined or treated to collect date for the primary outcome measure is May 2023. The estimated study completion date i.e., the date on which the last participant is examined or treated to collect final data for the primary outcome measures, secondary outcome measures, and adverse events is September 2025.
Promising preclinical data for VOR33
In preclinical studies published in PNAS, Vor Biopharma observed that CD33 removal by CRISPR-Cas9 provided robust protection of VOR33 HSCs from the cytotoxic effects of CD33-directed therapies, without deleterious effects on the differentiation or function of hematopoietic cells. The company announced FDA clearance of its investigational new drug (IND) application for VOR33 in January of this year, and presented data on successful scale-up of GMP-like VOR33 manufacturing at the American Society of Gene & Cell Therapy (ASGCT) Annual Meeting in May.
For a complete overview of current gene editing clinical therapeutic trials as well as diagnostic trials, check out CRISPR Medicine News' Clinical Trials Database.
Tags
Articlein vivoAcute Myeloid Leukemia, AMLCancerCancerCRISPR-CasCas9TrialsClinical
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.







