CMN Weekly (15 March 2024) - Your Weekly CRISPR Medicine News
By: Gorm Palmgren - Mar. 15, 2024
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...
Top picks
- American researchers have developed a novel non-viral nanoparticle delivery system for CRISPR-Cas9, targeting the FOXC1 gene in aggressive basal-like breast cancers (BLBC). The system utilises a pH-responsive amino phospholipid nanoparticle, facilitating efficient CRISPR-Cas9 delivery and gene editing in vitro and in vivo. An EpCAM aptamer enhances targeting toward BLBC cells, reducing off-target effects.
- Danish researchers have used CRISPR-Cas9 to target five tumour suppressor genes in mouse models of prostate cancer, preventing metastasis. The KMT2C gene - encoding a histone methyltransferase that epigenetically regulates gene transcription - was identified as a metastatic driver by regulating a specific gene cluster associated with ODAM and CABS1, which is essential for both tumour growth and the formation of metastases. The finding comes on the heels of two recent studies from Spain and the USA that have identified PRMT7 and CITED2, respectively, as critical regulators of prostate cancer metastasis.
Research
- Sangamo Therapeutics has engineered an adeno-associated virus (AAV) capsid that reaches 700-fold higher transgene expression in the brain than the competition. Preclinical data in non-human primates (NHPs) show that the novel capsid, STAC-BBB, showed robust penetration of the blood-brain barrier (BBB) and strong transgene expression throughout the central nervous system following intravenous administration.
- Researchers in the USA have presented a high-throughput prime editing sensor strategy that couples prime editing guide RNAs with synthetic versions of their cognate target sites to assess the functional impact of endogenous genetic variants quantitatively. Screening of over 1,000 endogenous cancer-associated variants of TP53 led to the identification of alleles that impact p53 function in mechanistically diverse ways.
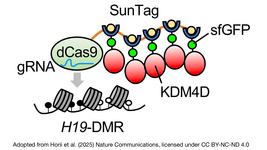
- Japanese researchers have developed CRISPRδ, a refined gene-editing tool designed to silence gene expression at the translational stage. The novelty of CRISPRδ lies in its approach to gene silencing. Rather than degrading the target RNA, it prevents translation by creating a steric hindrance for the ribosomes, thereby mimicking natural regulatory mechanisms without the collateral activity and toxicity associated with conventional Cas13 use.
- A genome-wide CRISPR screen has identified protein arginine methyltransferase 5 (PRMT5) as a molecular target in CDK4/6 inhibitor-resistant estrogen receptor-positive (ER+) and RB-deficient breast cancer. Treatment with the PRMT5 inhibitor pemrametostat and a selective ER degrader fulvestrant synergistically inhibits the growth of ER+/RB-deficient cell-derived and patient-derived xenografts.
- A new technique called CRISPR-Assisted mRNA Fragment Trans-splicing (CRAFT) employs CRISPR-Cas13 to enable the insertion of large RNA fragments into pre-mRNA transcripts. This method surpasses previous spliceosome-mediated approaches in editing efficiency for extensive segments across various mammalian cell lines.
- American research reveals that Cas12a's activation for DNA cleavage involves two steps: quick target binding followed by a slower allosteric transition, which does not necessarily require a protospacer-adjacent motif (PAM). Factors like DNA sequence, topology, length, and the presence of non-specific DNA or RNA can significantly influence activation times, offering new insights into Cas12a's function in bacterial immunity and its application in diagnostics.
- CRISPR-M is a new tool for predicting the sgRNA off-target effect using a multi-view deep learning network. It takes advantage of convolutional neural networks and bidirectional long short-term memory recurrent neural networks to construct a three-branch network for multi-views.
- A gene-editing approach using CRISPR-Cas9 and AAV6 shows promise in treating X-linked agammaglobulinemia (XLA) - a life-threatening immune disorder - by restoring BTK gene function in hematopoietic stem and progenitor cells (HSPCs). In vitro and in vivo experiments demonstrated the rescue of B cell maturation and immunoglobulin production, suggesting gene editing's potential as a definitive therapy for XLA, with efficiencies over 30% in human HSPCs.
Industry
- In a BioBusiness brief, representatives of the European Medicines Agency (EMA) provide guidance for developers of genome-editing medicinal products (GEMPs), like CRISPR-Cas9 therapies. Key challenges include quality control, off-target toxicity, and clinical trial design for rare diseases. The EMA encourages early dialogue through scientific advice to navigate regulatory frameworks. The approval of Casgevy, a CRISPR-based therapy for blood disorders, sets a precedent for GEMP regulation, highlighting the need for comprehensive preclinical and clinical evaluations.
- Allogene Therapeutics and Arbor Biotechnologies have announced a non-exclusive, global gene editing licensing agreement to use Arbor's proprietary CRISPR gene-editing technology in Allogene's next-generation AlloCAR T platform for treating autoimmune diseases.
- Poseida Therapeutics announced that the FDA has granted P-BCMA-ALLO1 Orphan Drug Designation for the treatment of multiple myeloma. The company's proprietary Cas-CLOVER and piggyBac technologies are used to produce P-BCMA-ALLO1, an off-the-shelf, allogeneic CAR-T product.
- Ricoh Company and ERS Genomics have announced a non-exclusive licensing agreement in the USA and Japan that gives Ricoh access to the foundational CRISPR-Cas9 genome editing technology patents managed by ERS Genomics. The agreement will contribute to expanding the creation of novel disease models.
2023 financial updates
- Caribou Biosciences has reported full-year 2023 financial results with a net loss of $35 million and $372 million in cash at the end of the year.
- Sangamo Therapeutics has reported full-year 2023 financial results with a net loss of $258 million and $81 million in cash at the end of the year.
- Allogene Therapeutics has reported full-year 2023 financial results with a net loss of $327 million and $449 million in cash at the end of the year.
- ProQR Therapeutics has reported full-year 2023 financial results with a net loss of $28 million and $119 million in cash at the end of the year.
Detection
- A new LbCas12a-based detection technique - CRISPR-Cas Autocatalysis Amplification driven by LNA-modified Split Activators (CALSA) - enhances DNA detection by combining split ssDNA activators with LNA-mediated site-directed trans-cleavage, creating an autocatalytic loop. This method achieves real-time, one-pot genomic and cell-free DNA detection with high sensitivity, specificity, and rapid response.
- Researchers in Thailand have developed a point-of-care CRISPR-Cas12a-based system (CRISPR-BP34) to detect the melioidosis-causing bacteria Burkholderia pseudomallei DNA across clinical specimens. The overall sensitivity of CRISPR-BP34 was 9% compared with 67% for culture-based detection.
- A new nucleic acid assay, Solid-Phase Extraction and Enhanced Detection Assay integrated by CRISPR-Cas12a (SPEEDi-CRISPR) negates the need for preamplification and significantly improves the detection of limit from the pM to fM level. Using Cas12a-coated magnetic beads, this assay combines efficient solid-phase extraction and enrichment of DNA targets enabled by the sequence-specific affinity of CRISPR-Cas12a with fluorogenic detection by activated Cas12a on beads.
Reviews
- Gene editing technology to improve antitumor T-cell functions in adoptive immunotherapy. This review presents recent representative findings on molecular insights into T-cell dysfunction and how genetic modification contributes to overcoming it. It also discusses several technical advances to achieve efficient gene modification using CRISPR and other novel platforms.
- CRISPR/Cas systems combined with DNA nanostructures for biomedical applications. This paper mainly reviews the design principles and biomedical applications of CRISPR-Cas combined with DNA nanostructures based on the types of DNA nanostructures.
- CRISPR-Cas-based diagnostic tools: Bringing diagnosis out of labs. This review aims to discuss the applicability of CRISPR-Cas-based diagnostic tools for infectious disease detection.
- Retron Library Recombineering: Next Powerful Tool for Genome Editing after CRISPR/Cas. This review discusses the current state of this brand-new tool that could eventually boost genome editing research.
- Therapeutic potential of CRISPR/CAS9 genome modification in T cell-based immunotherapy of cancer. This review explores the application of CRISPR technology in clinical trials and disease treatment, emphasising its role in enhancing CAR T-cell therapy for conditions like AIDS and cancer while addressing the associated challenges and potential for future advancements.
Perspectives & Webinars
- A feature in The Scientist explores how CRISPR leads the way in engineering the microbiome. It delves into the innovative use of CRISPR for manipulating entire microbiomes, highlighting its potential to revolutionise the study and treatment of various health conditions by targeting and editing the genomes of microorganisms within these complex ecosystems.
- "CRISPR screens decode human T cell function" is the title of a webinar produced by GenScript on Wednesday, April 17, at 11.00 EDT. Ralf Schmidt from the Medical University of Vienna will present his recent work, and Nikki Forrester from Nature Research Custom Media will moderate the event.
News from CRISPR Medicine News
- The program for CRISPRMED24 - our inaugural CRISPR Medicine Conference in Copenhagen, Denmark, April 23-25, 2024 - is now live. More than 300 scientists from academia and the industry have already signed up, and around 50 speakers and 100 poster presenters will share their most recent findings at the conference. Registration is still open, so don't hesitate to sign up.
To get more of the CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
CLINICAL TRIALS
IND Enabling
Phase I
Phase II
Phase III
Gastric Cancer and Colorectal Cancer, CRC, (NCT07166263)
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
IND Enabling
Phase I
Phase II
Phase III
Relapsed or Refractory Acute Myeloid Leukemia, AML, (NCT06541444)
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
IND Enabling
Phase I
Phase II
Phase III