CMN Weekly (3 May 2024) - Your Weekly CRISPR Medicine News
By: Gorm Palmgren - May. 3, 2024
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...
Top picks
- A new study by Belgian and other European scientists demonstrates that prime editing can effectively correct cystic fibrosis-causing CFTR mutations, specifically L227R and N1303K, in human organoids and airway epithelial cells. The prime editing approach achieved up to 34% genomic CFTR correction in rectal organoids derived from cystic fibrosis patients, resulting in up to 80% of the organoids showing functional rescue. The method shows promise for treating cystic fibrosis mutations not addressable by current therapies, offering a potential pathway to exact genomic medicine applications.
- Researchers have developed CRISPR-Cas9-engineered mouse models with mutations in Tourette disorder (TD) genes, CELSR3 and WWC1. These models exhibited typical TD behaviours such as sensorimotor gating deficits and repetitive motions, with variations between sexes. Aripiprazole treatment alleviated some symptoms. The models also showed enhanced dopamine release and reward learning, providing valuable insights into TD's neurobiology and potential therapies.
Research
- American scientists have developed the in4mer Cas12a platform, enabling large-scale genetic interaction screens in mammalian cells with arrays of four guide RNAs. The platform, using the Inzolia genome-scale library, is ~30% smaller than conventional CRISPR-Cas9 libraries but covers ~4000 paralog pairs. The new platform allows for the efficient discrimination of essential genes and detection of synthetic lethal interactions, offering a fivefold reduction in library size and cost compared to existing methods.
- Researchers in China have developed a guanidinobenzol-rich polymer that encapsulates CRISPR-Cas9 ribonucleoproteins (RNPs) into nanoparticles, enhancing genome editing efficiency from 30% to 70%. This system facilitates rapid endosomal escape and efficient delivery, both locally and systemically, significantly reducing PCSK9 levels in vivo and expanding the therapeutic potential of CRISPR-Cas9.
- A new study has successfully used base editing screens to uncover phosphorylation site dynamics and better understand how post-translational phosphorylation regulates T-cell functions. The research employed a pioneering CRISPR base editing strategy to alter specific phosphorylation sites that impact NFAT transcriptional activity, a pivotal pathway in T cell activation, and evaluate their functionality through high-throughput screens.
- Scientists in America have explored the molecular mechanisms behind the expanded PAM recognition capabilities of SpRY-Cas9, a CRISPR-Cas9 variant. They used structural and biochemical methods to demonstrate how SpRY-Cas9's conformational flexibility within its PAM-interacting region allows it to bind diverse DNA sequences, albeit with a slower activation rate and increased off-target risks. This research positions SpG-Cas9 as an intermediary variant with less flexibility than SpRY-Cas9.
- Research has identified that the microhomology-mediated end joining (MMEJ) pathway is central to large deletions (LDs) in CRISPR-Cas9 editing. Modulating POLQ and RPA, either genetically or pharmacologically, significantly affects these LDs. Lowering POLQ reduces LDs while adjusting RPA levels influences LD frequency. Also, inhibiting POLQ and adding recombinant RPA proteins improves homology-directed repair (HDR) in key cell types, offering strategies for more precise and safer genome editing.
- A Korean study has explored PDK1's role in osimertinib resistance in NSCLC linked to the EGFR C797S mutation. CRISPR-mediated knockout of PDK1 in resistant NSCLC cell lines curbed tumour growth in xenograft models, highlighting a potential therapeutic approach to tackle resistance in EGFR mutation-driven cancers.
- Italian researchers have identified CoCas9, a new compact CRISPR-associated protein from the human microbiome that shows promise for efficient and precise genome editing. CoCas9, a 1004-amino acid protein derived from an uncultivated Collinsella species, stands out due to its small size and high editing efficiency, making it particularly suitable for AAV delivery systems. The study demonstrated that CoCas9 could be efficiently packaged with its gRNA into an all-in-one AAV vector, achieving effective in vivo editing in mouse models.
- An American study introduces "Myospreader," a peptide sequence enhancing Cas9 delivery across multiple myonuclei in skeletal muscle. This improvement is crucial for effective CRISPR-Cas9 gene editing, as demonstrated in models of myotonic dystrophy and Duchenne muscular dystrophy. Myospreader-enhanced Cas9 shows increased protein stability and efficacy in gene editing, suggesting a significant advancement for nuclear-targeted gene therapies.
- A new method allows for high-efficiency precision genome editing with CRISPR in iPSCs, reducing the effort and time required to create isogenic lines. The method uses a combination of p53 inhibition and pro-survival small molecules to achieve homologous recombination rates higher than 90% in human iPSCs.
- American researchers showcase a miniaturised CRISPR-Cas12f system delivered via AAV. This system achieves effective gene editing across various cells and organs with minimal off-target effects, enhancing the potential for in vivo CRISPR gene therapy.
Industry
- Prime Medicine announced that the FDA has cleared its investigational new drug application for PM359 for the treatment of chronic granulomatous disease (CGD), a rare, inherited immune deficiency characterised by increased susceptibility to bacterial, mycobacterial and fungal infections. This move makes PM359 the first prime editor to enter the clinic and enables Prime Medicine to initiate its global Phase 1/2 clinical trial in the United States.
- Poseida Therapeutics and Astellas Pharma have entered into a research collaboration and license agreement to develop novel allogeneic cell therapies in oncology. The agreement will combine Astella's ACCELTM platform with Poseida's cutting-edge genetic editing platforms to further deliver innovative CAR-T cell therapies to cancer patients.
- Editas Medicine announced a two-year extension to its collaboration with Bristol Myers Squibb. Under this agreement, the parties may research, develop, and commercialise autologous and allogeneic alpha-beta T cell medicines using in vivo gene editing for the treatment of cancer and autoimmune diseases.
Detection
- A recent study enhances CRISPR-Cas12a-based nucleic acid detection by integrating water-in-oil-in-water (W/O/W) double emulsion (DE) droplets with flow cytometry, overcoming the stability issues of traditional single emulsion droplets. This method provides high-throughput, accurate quantification of human papillomavirus type 18 (HPV18) DNA, showcasing the DE platform's potential for precise biomarker analysis.
- A new synthetic biology cascade reaction on a paper substrate uses CRISPR-Cas12a and recombinase polymerase amplification (RPA) to enable the programming of a standard toehold RNA switch for a genome of choice. The sensor exhibits high sensitivity and is capable of visually detecting as few as 100 copies of the whole genome from a model Salmonella pathogen on a paper substrate.
- A new CRISPR-Cas12 assay can detect a specific lung cancer mutation (exon 19 deletion in the epidermal growth factor gene), achieving high precision by analysing Cas12 trans-cleavage kinetics. After optimising variables across 780 reactions, the assay demonstrated 99% accuracy on 18 tumour samples.
- Recent advancements in CRISPR-Cas tools have heightened concerns about gene doping in sports. A study improved an RT-RPA-based detection method using SHERLOCK for identifying sgRNA associated with Streptococcus pyogenes in serum, enhancing sensitivity from 100 pM to 1 fM. This method, validated in mouse models, offers a promising new approach for detecting illicit CRISPR-Cas doping, demonstrating potential in future anti-doping strategies.
- A rapid, sensitive, and user-friendly method for the detection of Pseudomonas aeruginosa is based on recombinase polymerase amplification (RPA) and CRISPR-Cas12a. The process targets specific regions within the lasB gene and offers a limit of detection (LOD) of 100 copies/µL.
- Cas13a-SDT is a new point-of-care diagnostic for asymptomatic malaria, achieving WHO sensitivity standards, which leverages dual Cas13a trans-cleavage and strand displacement-triggered transcription. The method, tested on clinical samples, provides specific, accurate, and user-friendly results comparable to RT-PCR, supporting its potential to enhance malaria control efforts.
Reviews
- Gaining momentum: stem cell therapies for HIV cure. This review focuses on the development of stem cell therapies, including gene-editing techniques like CRISPR-Cas9, to achieve durable ART-free HIV-1 remission without relying on stem cell transplants.
- Biomaterial-Based CRISPR/Cas9 Delivery Systems for Tumor Treatment. This review focuses on the development of biomaterial-based delivery vectors for CRISPR-Cas9, aiming to enhance its application in clinical oncology, particularly for treating tumours with complex genetic backgrounds.
- CRISPR Advancements for Human Health. This review provides a comprehensive overview of CRISPR and its clinical applications. It introduces the CRISPR system and explains how it works as a gene editing tool.
News from CRISPR Medicine News
- Last week, CRISPR Medicine News saw the very successful holding of its inaugural CRISPRMED24 conference, the first-ever CRISPR medicine conference in Europe. We have written a wrap-up of the meeting and disclosed the names of the eight CRISPRMED24 Poster Prize winners and the three winners of the CRISPRMED24 Exhibitor Quiz. Moreover, we share the names of the ten lucky CRISPRMED24 Travel Grant awardees who got a travel grant for the conference generously sponsored by MaxCyte - a leader in cell engineering and innovation of tomorrow's technological solutions.
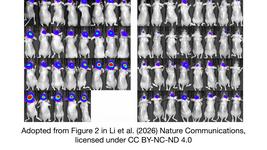
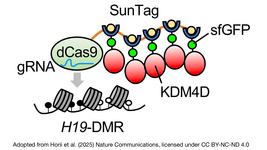
- On Monday, we interviewed Italian researchers who have addressed the critical need for experimental validation of durable epigenetic silencing after transient delivery of editors in vivo. Using lipid nanoparticles loaded with editor mRNAs, they showed that a single administration led to a significant reduction in circulating Pcsk9 levels in mice for nearly a year.
- On Wednesday, we announced that the FDA has cleared the first clinical trial application for a prime editor, Prime Medicine's investigational drug PM359, for the treatment of chronic granulomatous disease (CGD), a rare, inherited immune deficiency characterised by increased susceptibility to bacterial, mycobacterial, and fungal infections.
To get more of the CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
CLINICAL TRIALS
IND Enabling
Phase I
Phase II
Phase III
Gastric Cancer and Colorectal Cancer, CRC, (NCT07166263)
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
IND Enabling
Phase I
Phase II
Phase III
Relapsed or Refractory Acute Myeloid Leukemia, AML, (NCT06541444)
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
IND Enabling
Phase I
Phase II
Phase III