CRISPR and stem cells to cure diabetes
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...

In a molecular and cell transforming engineering feat researchers demonstrate for the first time a potential cure for genetic forms of diabetes. They take patient skin cells, turn them into stem cells, use CRISPR-Cas9 gene editing to correct a diabetes-causing mutation, and then transform the edited cells into the insulin-producing beta cells the patient lacks. Transplanting the beta cells into severe diabetic mice rapidly reverses their diabetes, providing a robust and functional cure.
»This technology now allows the potential use of gene therapy in combination with patients' own cells to treat their diabetes by transplantation of lab-grown beta cells,« says assistant professor Jeffrey Millman, Washington University School of Medicine, US.
He led the study in collaboration withphysician-scientist Dr. Fumihiko Urano, who treats patients with a disease called Wolframs syndrome.
»I have seen many patients of Wolframs syndrome, and I have been looking for a treatment for this devastating disorder. So this is great news,« says Urano.
The researchers acknowledge that more preclinical work is needed. Still, the therapeutic approach raises the possibility to cure Wolframs syndrome and other forms of diabetes caused by mutations in a single gene.
Diabetes is a loss of beta cells
Diabetes affects hundreds of millions of people globally, and the disease is caused by the loss or dysfunction of the insulin-producing beta cells in the pancreas. Functional loss of beta cells is generally treated with insulin injections, but long-term complications can arise.
Wolframs syndrome
Wolframs syndrome, also called DIDMOAD (diabetes insipidus, diabetes mellitus, optic atrophy, and deafness), is a rare autosomal-recessive genetic disorder that causes childhood-onset diabetes mellitus and quickly require insulin-replacement therapy - insulin injections multiple times each day. Most go on to develop problems with vision and balance, as well as other issues, and in many patients, the syndrome contributes to an early death.
Wolfram syndrome occurs in approximately 1/500,000 people but there is evidence it occurs with far greater frequency in certain populations.
To have Wolframs syndrome an individual must have a mutation in both of his or her copies of the WFS1 gene that codes for wolframin protein. Wolframin protein helps fold other proteins properly and maintain proper calcium levels in the endoplasmic reticulum.
Transplantation of beta cells could be a promising alternative to insulin injections at least for some diabetes patients. And with the 2012 Nobel-prize winning discovery of induced pluripotent stem cells (iPSC), it is potentially possible to create unlimited numbers of beta cells for diabetes cell therapy. iPSCs are stem cells that can be transformed into any of the cell types found in the body if given the correct cocktail of growth factors and nutrients.
Jeffrey Millman's lab focuses onusing stem cells forthe study and treatment of diabetes, and over the years has successfully developed new strategies to differentiate iPSCs into beta cells.
Four years ago, Millman moved to Washington University School of Medicine and partnered with Dr. Urano, who wanted to use the technology to treat patients with Wolframs syndrome.
Patients with Wolframs syndrome develop diabetes in childhood as well as other ailments, including vision loss and balance issues due to optic nerve atrophy and neurodegeneration. The disease is a rare recessive disorder caused by mutations in one gene, WSF1.
Combining stem cells and CRISPR
They decided to combine the CRISPR-Cas9 gene editing method with the iPSC stem cell technology to develop a treatment for Dr. Uranos patients using their own cells.
Here is how they did it. First, they took skin cells from three patients and turned the cells into iPSC stem cells using a cocktail of growth factors and nutrients. Then applied CRISPR-Cas9 gene-editing to correct a single nucleotide mutation in the WSF1 gene generating three clonal iPSC stem cell-lines.
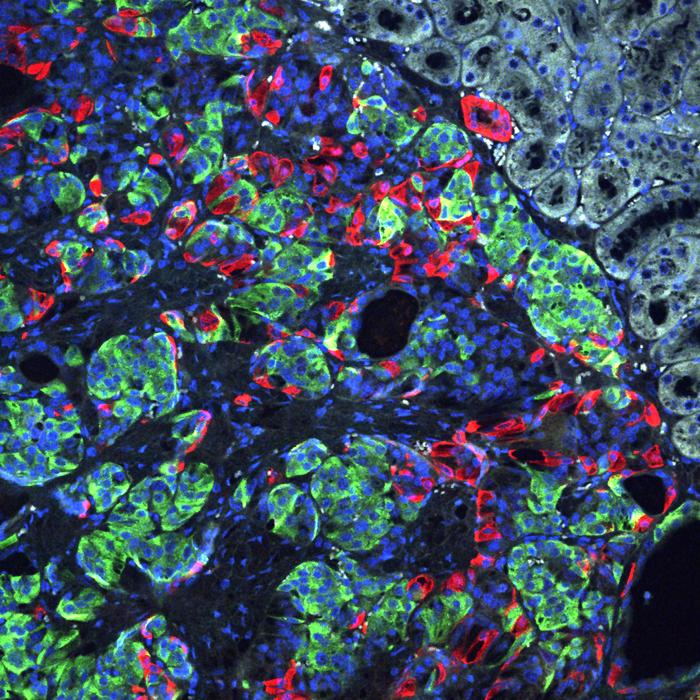
Finally, they turned to new cocktails of growth factors and nutrients targetting several developmental pathways to make the iPSC stem cells differentiate into insulin-producing beta cells.
As a control they also turned patient-derived cells that had not been genetically modified into beta cells.
The unedited beta cells were also able to produce insulin, but at a much lower level. Measuring single-cell RNA sequences from several thousand edited and unedited beta cells, they observed major defects in the unedited beta cells, including very weak insulin secretion and many markers of stress and dysfunction.
»Amazingly this is all fixed in the gene-corrected beta cells, and they were indistinguishable from beta cells that we made from healthy donors,« says Jeffrey Millman.
Curing diabetic mice
For the final test, the researchers injected the CRISPR-corrected 'beta cells' into severely diabetic mice. These animals were rendered diabetic by a toxin injection (streptozotocin), which destroys the endogenous beta cells.
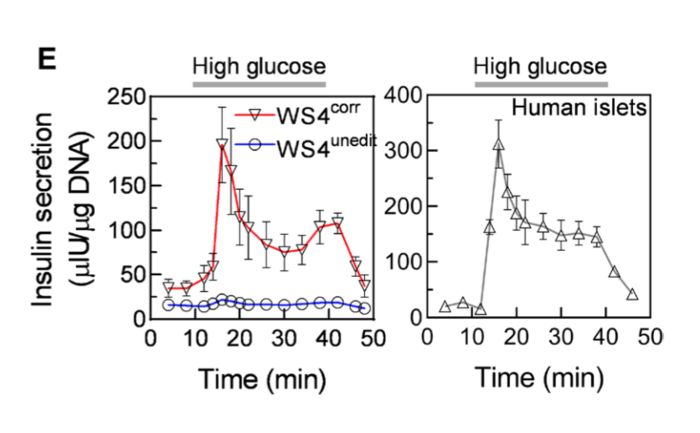
As controls, they injected unedited 'beta cells' and beta cells from human cadavers.
The edited 'beta cells' were superior to the unedited beta cells. They maintained stable insulin and blood glucose numbers for the remainder of the observation period up to six months.
»This is the first demonstration of the translational potential of patient-derived stem cells, let alone with CRISPR-correction of a diabetes-causing mutation. It now opens up the potential of using a patient’s own cells to treat their diabetes,« says Jeffrey Millman.
Off-targets not a big concern
One major concern of gene editing therapies is the risk of off-target mutations, which potentially can cause cancer.
»Fortunately, we did not observe any off-targets,« says Jeffrey Millman.
The researchers computationally predicted the five most likely genomic locations and sequenced those without finding any mutations.
Secondly, they saw no adverse effects in the mice after six months that would indicate off-targets, such as uncontrolled growth.
In translating the approach to humans, Jeffrey Millman doesn't believe off-target will be a significant safety risk, especially since the cells are edited ex vivo.
»I would imagine performing whole exome sequencing of gene-edited cells to confirm lack of off-targets in the protein-coding region of the genome. This would allow us to select only clones with the correct gene edits without off-targets, making off-targets in my opinion not a major concern in this context,« he says.
VIDEOFirst-author Kristina Maxwell and associate professor Jeffrey Millman at Washington University School of Medicine discusses the importance of their new discoveries, the context to the field, challenges, and ethical concerns. Credit: Washington University School of Medicine
Future perspectives
In summary, the research is a proof-of-concept that monogenic forms of diabetes can be cured by correcting the diabetes-causing mutation in stem cells derived from patients' own cells.
Millman is looking into two avenues for future work.
One is to use thesystem as a valuable disease-on-a-dish model of diabetes.
»By having patient-derived cells with and without diabetes-causing mutations, we can recapitulate key aspects of diabetes pathology in the lab. This enables a new platform for us and the field to test new drug interventions to prevent, stop, or reverse diabetes pathology,« says Millman.
The second avenue they are working on is the exciting prospect of a therapy for patients with Wolframs syndrome in the near future using their own cells.
»Our hope would be to put the cells in a type of removable cage and place them under the skin,« says Jeffrey Millman.
And perhaps in time, the research could pave the way for treatments for monogenetic diseases beyond diabetes.
VIDEO: Dr. Fumihiko Urano, professor at Washington University, discusses the clinical implications of the study: Gene editing-based treatments for Wolfram syndrome and diabetes.
Credit: Fumihiko Urano, MD, PhD, Professor at Washington University.
Tags
ArticleInterviewDiabetesMetabolic disorderGene therapyCRISPR-Cas
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.









