CRISPR editing links an FTO variant to muscle insulin resistance
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...
The researchers generated isogenic cell lines differing only at this SNP and directed their differentiation into mesodermal, endodermal, and neuroectodermal progenitors (see Figure 1). Among the five tested lineages, skeletal muscle cells exhibited the most pronounced effects, with increased proliferation, differentiation, and metabolic ageing.
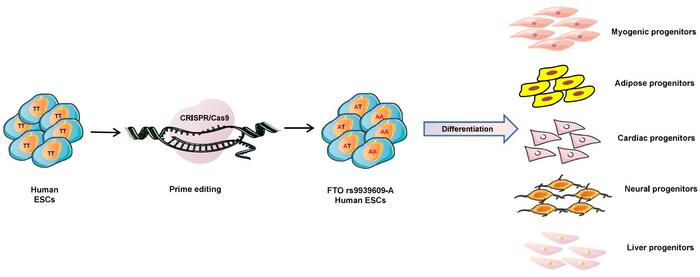
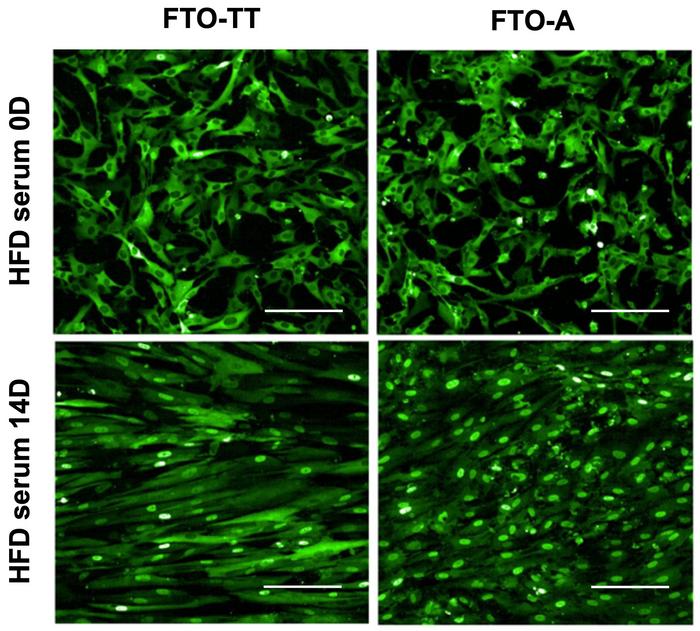
Muscle progenitors carrying the rs9939609-A allele showed enhanced activation of the insulin/IGF signalling pathway, leading to initial increases in insulin sensitivity. However, long-term culture or exposure to a high-fat diet (HFD) resulted in the development of insulin resistance. This transition was visualised using a FoxO1-GFP reporter system, which tracks insulin signalling by monitoring the localisation of the FoxO1 protein.
In insulin-sensitive cells, FoxO1 remains in the cytoplasm, but in FTO rs9939609-A myotubes exposed to HFD serum, FoxO1 progressively translocated to the nucleus, indicating insulin resistance (see Figure 2). This shift was accompanied by decreased phosphorylation of key insulin pathway proteins, confirming a loss of insulin responsiveness.
The study demonstrates that the FTO rs9939609-A allele enhances insulin and IGF signalling by increasing FTO expression and reducing m6A methylation on H19 lncRNA and IGF2 mRNA. CRISPR-edited muscle cells showed overactivation of the FTO-H19/IGF2 regulatory circuit, driving initial muscle growth and heightened insulin responsiveness but ultimately leading to insulin resistance with age or dietary stress.
By isolating the effects of a single genetic variant, this CRISPR-based approach clarifies how FTO polymorphisms influence muscle metabolism and their potential contribution to metabolic disease.
This research was led by Lu Guang and Ng Shyh-Chang at the Chinese Academy of Sciences, Beijing, and it was published in Nature Communications on Friday, 7 March 2025.
Meet the leading scientists working within the delivery field at CRISPRMED25 - The 2nd CRISPR Medicine Conference in Copenhagen, April 8-11, 2025.
To get more CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
ArticleCMN HighlightsNewsDiabetesType 2 diabetesPrime editors
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.







