CRISPR Fights Diabetes and Obesity by Changing the Fate of Fat Cells
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...

Silvia Corvera and Michael Czech are confident. They have spent decades researching type 2 diabetes, trying to find new therapies for the metabolic disease that hits up to 10% of the population. Recently, they achieved promising results for a CRISPR-based cell therapy in mice. And clinical trials are the longer-term goal thanks to a clever use of the gene-editing technology that reduces the risk of unwanted side effects.
»We are very excited about the technology. We have shown proof of concept in mice, and this has given confidence to us and our funders to move ahead in non-human primates already this year,« says Michael Czech, Professor in Molecular Medicine at the University of Massachusetts Medical School and co-senior author of the study that was published in Nature Communications.
The new therapy is based on the implantation of fat tissue that has been gene-edited to burn fat and glucose and secrete various factors that regulate metabolic health. Silvia Corvera, MD, and Endowed Chair in Diabetes Research, also at the University of Massachusetts Medical School, is the study’s other co-senior author. She explains that current medications for diabetes tend to focus on controlling glucose levels. But the situation is much more complex.
»People with diabetes or obesity develop many comorbidities and complications that are not fixed after controlling glucose levels. For example, they might have developed fatty liver disease, heart disease, kidney disease, retinal disease, and susceptibility to infections. It is not just one thing, but a much bigger conglomerate of issues. And current thinking suggests that adipose tissue dysfunction contributes to these complications. So, we thought that putting functional fat into patients would be much more likely to result in improvements in metabolic diseases,« she says.
“While we believe that existing and upcoming medications will improve our ability to prevent and manage diabetes, the advent of safe and effective gene editing may provide innovative therapeutic options for people with diabetes”Marlon Pragnell, American Diabetes Association
Marlon Pragnell, Vice President of Research and Science for the American Diabetes Association, welcomes the new approach and is cautiously optimistic about gene editing in the treatment of diabetes:
»While we believe that existing and upcoming medications will improve our ability to prevent and manage diabetes, the advent of safe and effective gene editing may provide innovative therapeutic options for people with diabetes.«
A single gene affects the fate of fat cells
Brown fat is key to the new therapy. This kind of adipose tissue was first discovered in humans less than 20 years ago, and it has some remarkable features not found in the much more common white fat. For one, brown fat cells express uncoupling protein 1 (UCP1), an enzyme that leads to dissipation of energy as heat instead of ATP after oxidative phosphorylation in the mitochondria. Moreover, they secrete several factors favourable to metabolic homeostasis. Brown fat cells are typically interspersed with white fat cells in so-called beige adipose tissue.
»People with diabetes have literally no beige fat, and as obesity increases, there is a trend towards decreased beige fat. This correlates very well with metabolic health. So the big question is: why do people with more beige fat generally have better metabolic health?« asks Silvia Corvera. And Michael Czech has a key to the answer:
»Back in 2004, we realised that when a gene called Nrip1 was knocked out in fat cells, they responded much better to insulin. Over the years, it became clear that Nrip1 suppresses insulin sensitivity and many of the genes usually expressed in brown fat cells, including Ucp1, that causes them to burn fat. So basically, Nrip1 is a master regulator that suppresses the formation of the healthy brown adipose tissue.«
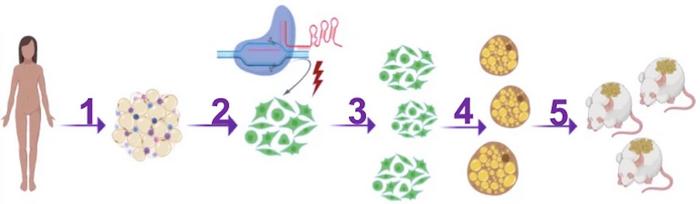
The connection between Nrip1, brown fat, diabetes and obesity prompted Silvia Corvera and Michael Czech to reach out for gene editing as a new approach to treating diabetes. They reasoned that by knocking out Nrip1 in human preadipocytes, i.e., fat cell precursors, they would differentiate into brown fat cells rather than become white fat. And if edited cells were subsequently transplanted into mice (or eventually a person), their ability to burn fat and glucose and secrete metabolic factors could lead to metabolic health benefits.
The CRISPR strategy is future proof
The two senior researchers and their team at the University of Massachusetts Medical School set out using CRISPR-Cas9 and a selection of guide RNAs (gRNAs) that target the coding region of Nrip1 in human preadipocytes in vitro. However, since the researchers were determined to eventually translate their approach into a clinical therapy for diabetes and obesity, they had to keep that in mind from the very beginning.
»We wanted to achieve a strategy where the CRISPR reagents would only be present in the cells for just the time needed to edit the target. Then the Cas9 and gRNAs should be degraded and thereby minimise the risks of immunogenic reactions and off-target effects caused by sustained activity,« explains Michael Czech.
“By omitting a vector and instead delivering Cas9 as a protein which is only present for a limited time, the authors elegantly achieve the wanted gene editing effect but also greatly reduce the risk of off-target effects”Morten Lundh, Gubra, Denmark
To accomplish this, the team decided to electroporate cells with Cas9 protein and gRNA in the form of ribonucleoprotein complexes. They are rapidly degraded in cells, and the direct in vitro administration of CRISPR reagents ensures that no other cell types other than the desired preadipocytes are targeted. After gene editing of human preadipocytes, no off-target editing was detected using the tools GUIDE-seq or Cas-offinder.
Morten Lundh, a project leader at Gubra, Denmark, working with pre-clinical development for metabolic diseases, acknowledges this approach for delivering CRISPR reagents. He was not involved in the study but has previously used CRISPR to engineer human brown-like adipocytes to ameliorate obesity and metabolic syndrome in mice.
»By omitting a vector and instead delivering Cas9 as a protein which is only present for a limited time, the authors elegantly achieve the wanted gene editing effect but also greatly reduce the risk of off-target effects. From a clinical point of view, off-targets must be avoided, and the authors have demonstrated one way to achieve this,« says Morten Lundh.
Gene knockout improves metabolic health
CRISPR gene editing of human preadipocytes led to disruption of Nrip1 in around 80% of cells, with indel profiles showing that around half of the edited genes had either 1-bp deletions or 1-bp insertions. These numbers were sustained as cells differentiated into mature adipocytes. Using the best performing gRNA, H5, gene editing resulted in significant depletion of NRIP1 protein after seven days, and this was associated with an 80-fold upregulation of Ucp1 expression.
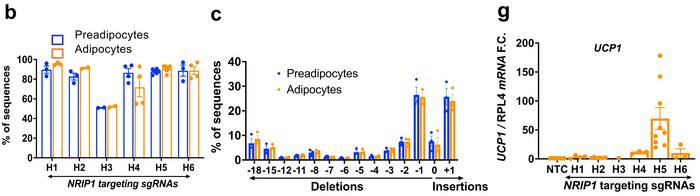
The knockout of Nrip1 had significant consequences for the expression of several other genes, most of which were upregulated. This was expected since Nrip1 is known to be a suppressor gene. RNA-seq analyses showed that a large portion of the upregulated genes was related to thermogenesis, mitochondrial respiration, and fatty acid oxidation. Moreover, the ProFAT computational tool indicated that Nrip1-knockout adipocytes had a much greater brown-like probability than unedited cells.
»These adipocytes are now more brown because the suppressor gene has been knocked out. So, they are burning more glucose, they are burning more fat, as well as secreting various factors,« Michael Czech says.
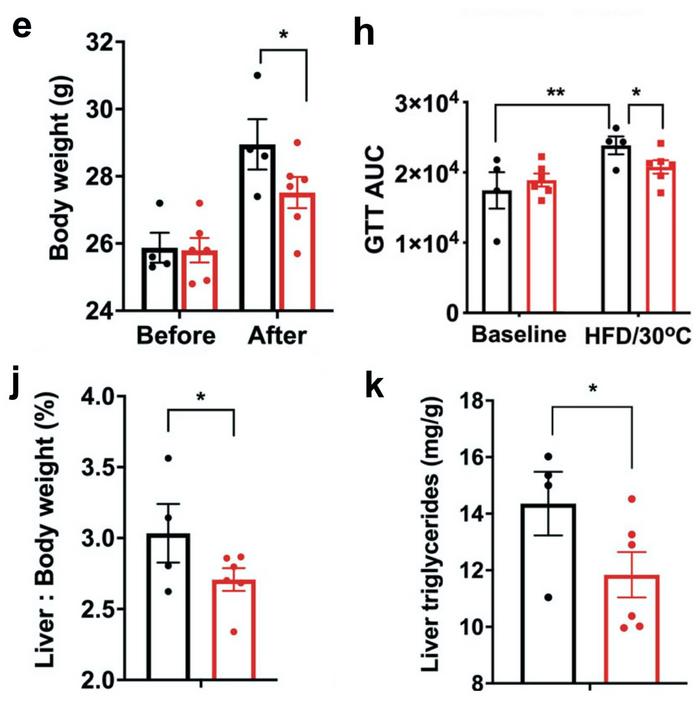
To test the physiological effect of this, the researchers implanted CRISPR-modified human adipocytes under the skin of obese, glucose-intolerant and immunocompromised mice. In contrast, controls were implanted with mock-edited adipocytes. The mice were fed a regular CHOW diet for ten weeks after transplantation, and during this period, no difference in body weight was observed between the control and experimental group.
However, three weeks after a shift to a high-fat diet, mock-treated mice had gained significantly more weight (around 5%) than mice transplanted with Nrip1-knockout adipocytes. In addition, after the change of diet, control mice displayed a significant decrease in glucose tolerance, while this was not the case in experimental mice transplanted with CRISPR-edited adipocytes. Moreover, two other indicators of metabolic health - relative liver to body weight ratios and liver triglycerides - also developed significantly more favourably in the experimental mice.
More genes need to be knocked out
Morten Lundh acknowledges the results but questions the choice of gene target for editing:
»Although the authors show promising effects of knocking out Nrip1 in human adipocytes, this may not be the best candidate going forward from a translational point of view. However, applying the concept of transient Cas9 protein delivery to target a protein with fewer potential dramatic side effects would be very interesting.«
Michael Czech, on the other hand, believes that the way forward is to knock out additional genes along with Nrip1:
»The Nrip1 knockout brings the adipocytes part of the way to their browning, but they are not entirely brown. We think we can get the cells even more brown by knocking out additional genes. We are now screening other genes - and we have screened about 30 or 40 already - that in combination with Nrip1 may cause a much more extensive browning and therefore amplify the therapeutic effects we have already seen.«
“We now need to move from mice to non-human primates to see if what we have achieved so far will be enough for clinical relevance, and this work is already in progress”Silvia Corvera
»We now need to move from mice to non-human primates to see if what we have achieved so far will be enough for clinical relevance, and this work is already in progress. In addition, we need to make gene-edited cells that avoid immune surveillance, so we have an off-the-shelf cell product that isn't restricted to patients' own immune systems. We are, however, very optimistic about this because for some unknown reason, adipose tissue tends to be poorly immunogenic, and we can enhance this property with CRISPR-mediated modification of specific genes,” concludes Silvia Corvera.
Link to the original article in Nature Communications:
CRISPR-enhanced human adipocyte browning as cell therapy for metabolic disease
To get more of the CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
ArticleInterviewNewsin vivoElectroporationRibonucleoprotein (RNP)DiabetesObesityType 2 diabetesCas9
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.







