CRISPR gene therapy makes fat cells burn fat and prevents obesity in mice
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...

In 2015, a young Danish scientist moved to Boston in the US to do his postdoc at Harvard Medical School. During his studies in molecular biomedicine at the University of Copenhagen, Morten Lundh had become fascinated by the relatively newly discovered role of brown adipose tissue in humans.
In contrast to white adipose tissue, the brown tissue does not just passively store fat but has a thermogenic capacity to burn it as fuel and dissipate the energy as heat in response to cold or excess feeding.
At the Joslin Diabetes Center in Boston, Dr Yu-Hua Tseng had played a pioneering role in the discovery of human brown fat in 2009 and had developed animal models based on human tissue. Lundh saw an excellent opportunity to join her lab.
»The idea was to investigate if human white adipocytes - that make up the majority of fat cells in obese patients - and their progenitors could actively be used in the fight against obesity, by turning them into brown-like adipocytes. It now seems to be the case,« Morten Lundh says, referring to a paper the two scientists and their colleagues published today in the prestigious journal Science Translational Medicine.
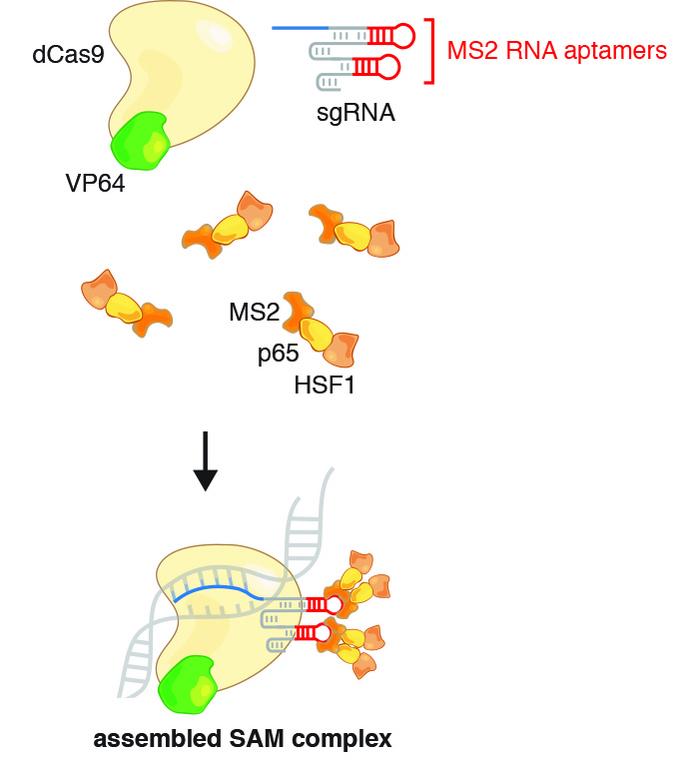
CRISPR-SAM activates genes without changing the genome
One of the main differences between brown and white fat cells is that the former express the uncoupling protein 1 (UCP1) gene at much higher levels.

The UCP1 gene product works at the mitochondrial inner membrane to uncouple the respiratory chain, so the proton gradient generated from oxidative phosphorylation is decreased, and chemical energy is dissipated as heat rather than being used to synthesise ATP.
Lundh and Tseng wanted to activate the dormant UCP1 gene in immature white fat cells, so they turned to the CRISPR Synergistic Activation Mediator (SAM) protocol, initially developed in 2014 by Feng Zhang at Massachusetts Institute of Technology. Elvir Becirovic from Ludwig-Maximilians-Universität München, Germany recently employed a similar approach to treat mice from hereditary blindness caused by retinitis pigmentosa.
CRISPR-SAM utilises a deactivated Cas9 (dCas9) and a modified gRNA to make a transcriptional activator interact with the regulatory domain of a desired gene and thereby facilitate transcription. In this case, the UCP1 gene was targeted and the procedure worked as expected, leading to a 6,000-fold increase in UCP1 mRNA and a 20-fold increase in UCP1 protein.
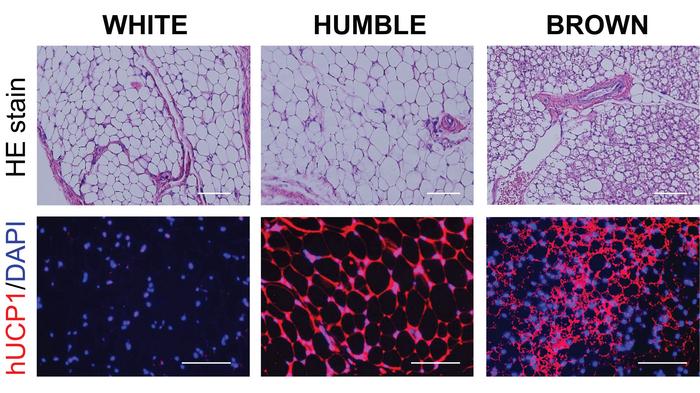
The scientists dubbed the transfected cells HUMBLE (human brown-like) cells since they not only expressed UCP1 as brown fat cells do but also showed altered expression levels of several other genes and a different phenotype than untreated white fat cells.
UCP1-activated HUMBLE cells make mice lose weight
These collateral effects were shown not to be due to off-target activations, Lundh explains: »Indeed, the HUMBLE cells have altered expression levels of several genes, and we believe that this is a direct effect of overexpressing UCP1. First of all, we did examine potential off-targets, identified using an algorithm, and they were not altered compared to untreated white fat cells.«
»In addition, we found that increased UCP1 expression leads to elevated AMP concentration and AMPK activation, which in turn upregulates expression of genes involved in mitochondrial biogenesis, such as PGC1a and NRF1,« adds Tseng.
The scientists believe that the forced overexpression of UCP1 is driving mitochondrial biogenesis to accommodate the increased UCP1 protein and thereby changing the fate of pre-adipocytes.
As the project moved forward, another postdoctoral scientist from the Tseng lab, Chih-Hao Wang, took over most of the research work. The next step was to multiply HUMBLE cells in the lab and transplant the transfected tissue onto white adipose tissue in mice to see if this affected diet-induced obesity.
It did, indeed. Mice with HUMBLE cells gained less weight and displayed up to 35 % improvements in glucose tolerance and insulin sensitivity compared to controls. However, one observation puzzled the scientists, as Lundh explains: »Interestingly, when we measured glucose uptake in mice with HUMBLE implants compared to mice implanted with control implants, we found the HUMBLE-implanted mice to take up more glucose, however not in the implants, but in the endogenous brown fat tissue. Thus, the observed metabolic effect is, at least with respect to glucose uptake, most likely mediated by the endogenous tissue.«
Fat depots talk to each other
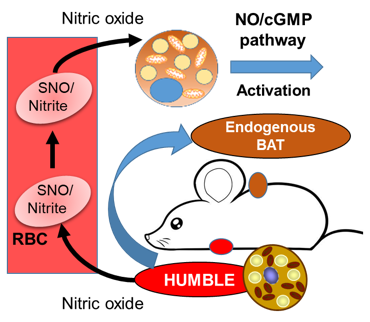
In other words, the HUMBLE cells send a signal to the endogenous brown fat cells that in turn, change their metabolic effect.
»The major challenge in this study was to delineate the cross-talk between the transplanted HUMBLE cells and the endogenous brown fat,« says Tseng and adds that the signal eventually turned out to be NO (nitric oxide) before passing the word over to Lundh:
»We found that HUMBLE cells actively secrete NO, which is a known vasodilator, thereby increasing blood flow to brown fat tissue and allowing it to utilise more fuel in the form of nutrients. Also, and others have demonstrated this, NO promotes mitochondrial biogenesis which subsequently results in brown fat tissue which efficiently will promote beneficial effects such as burning fat and generate heat.«
»This is an important finding that needs to be investigated further if HUMBLE cells should eventually be used in the clinical setting as a cell-based therapy,« says Tseng.
Lundh elaborates: »HUMBLE cells mediate their beneficial effects via NO release, and thus it is fair to reason that the effect is most likely systemic and will also have effects on other brown fat depots. Thus, from a translational point of view, it should be feasible to obtain systemic effects by transplanting enough HUMBLE tissue just once, assuming that NO released from the transplant reaches the blood circulation.«
In vitro CRISPR is best suited to the clinic right now
Morten Lundh explains that the approach with transplantation of in vitro CRISPR-treated cells is a favourable choice for human therapies: »One major advantage of our approach, compared to direct in vivo CRISPR treatment, is the possibility for tight control. We can easily control the number of cells treated with CRISPR and more importantly, we can evaluate their characteristics and thereby hopefully eliminate any unwanted effects which may appear in an in vivo setting.«
However, Tseng cautions that lentivirus is a retrovirus that integrates into the host genome and therefore can lead to unwanted off-target insertional mutagenesis.
»Since we used lentiviruses to deliver the CRISPR-SAM, for safety concerns, we engineered the cells in vitro and then transplanted them into the recipient animals. When a safe and specific virus-free gene delivery system is available, direct in vivo CRISPR treatment might be preferable,« says Yu-Hua Tseng.
Link to the original article in Science Translational Medicine: CRISPR-engineered human brown-like adipocytes prevent diet-induced obesity and ameliorate metabolic syndrome in mice.
Yu-Hua Tseng, PhD, a Senior Investigator in Joslin’s Section on Integrative Physiology and Metabolism discusses recent findings on brown fat research. This research finds a potential therapy for obesity using transplanted HUMBLE (human brown-like) fat cells - human white fat cells that have been genetically modified using CRISPR to become similar to heat-generating brown fat cells. CREDIT: Joslin Diabetes Center.
Tags
ArticleInterviewNewsLentivirus (LV)ObesityGene therapyTranscriptional activationOff-targetCRISPRa
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.








