CRISPR Provides New Hope for Control of Mosquito-Borne Viral Diseases
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...

Mosquitoes are among the most widespread and dangerous vectors of viral diseases in the world. Aedes mosquitoes transmit notorious arboviruses such as Zika virus, yellow fever, dengue fever and chikungunya virus, collectively infecting hundreds of millions and killing many thousands of people each year.
In a paper that was recently published in Nature Communications, a research group led by Dr. Omar Akbari at the University of California, San Diego, details how they engineered a CRISPR-based molecular genetic control system to suppress experimental populations of Aedes Aegypti.
Population suppression may eliminate mosquito-borne viruses because the insect vector is critical for viral transmission.
Traditional mosquito control strategies are primarily based on the use of chemical insecticides, which are expensive, harmful to the environment, and affect not only mosquitoes but also beneficial insects, e.g., honeybees, owing to their nonspecific nature.
Genetic control systems represent promising alternatives to better manage mosquito populations with fewer environmental concerns.
Genetic control with Sterile Insect Technique
Sterile Insect Technique (SIT) is the most successful genetic control system used to control insects to date. Here, male mosquitoes are separated from females and then irradiated, leading to sterilisation via random genetic mutations or chromosomal abnormalities. Sterile males are then released into the wild to mate with wild females (see Fact Box).
Despite its successes, SIT has its limitations, which prompted Dr. Omar Akbari and his research team to turn to CRISPR to develop a scalable system for mosquito population control:
»The fragile nature of mosquitoes and the required radiation doses often result in reduced fitness, limiting their ability to suppress the population and ultimately the effectiveness of the SIT. You need another way to both sex sort and sterilise them [mosquitoes] that is more specific«, explains Dr. Akbari.
Sterile Insect Technique
The principle behind sterile insect technique (SIT) is that sterile males will not produce progeny and will therefore help to suppress the insect population. SIT is considered safe despite the introduction of additional insects into the environment. Importantly, male mosquitoes neither bite nor transmit pathogens, in contrast to females. SIT has been successfully used to eradicate certain wild pest populations, including populations of the New World screwworm, the Tsetse fly, and the Mediterranean fruit fly.
Precision guided-SIT: leveraging CRISPR to reduce mosquito fertility
Dr. Akbari and his team took advantage of CRISPR to precisely target genes required for A. aegypti fertility without compromising fitness, effectively upgrading SIT to precision guided-SIT (pgSIT):
»We targeted a gene called β-Tubulin, which is important for fertility. Without that gene, the sperm does not become elongated, resulting in sterile males. Then we targeted a gene called myo-fem, which is important for female flight. Without this gene females cannot fly. And if they cannot fly, they cannot reproduce, they cannot find blood and they cannot transmit pathogens«, explains Dr. Akbari about the pgSIT approach and the rationale behind the chosen target genes.
To produce sterile males and flightless females, the team first generated transgenic gRNA-expressing mosquito lines using embryo microinjection protocols, where each line encoded four gRNAs targeting conserved sites in the β-Tubulin and myo-fem genes. These lines were subsequently crossed with a transgenic Cas9-expressing line to generate a transheterozygous progeny (gRNA/+; Cas9/+). This setup allowed the researchers to establish that β-Tubulin disruption results in male infertility but maintains fertility in females, while myo-fem disruption yields flightless females but does not affect flight capacity in males (see Figure 1).
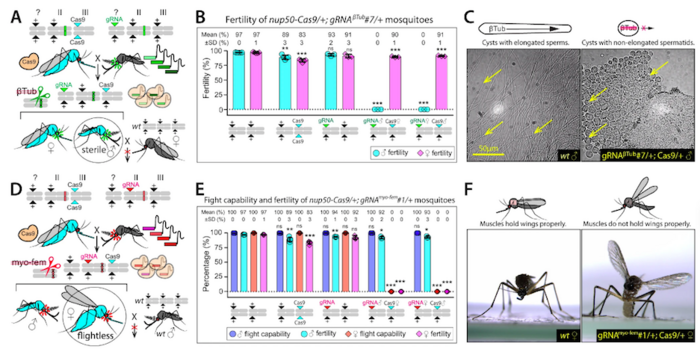
To generate a pgSIT line capable of targeting both β-Tubulin and myo-fem simultaneously, two gRNA lines that exclusively produced sterile males (gRNAβ-Tub) or flightless females (gRNAmyo-fem) were combined to generate a double-homozygous stock (gRNAβ-Tub+myo-fem). Subsequently, the gRNAβ-Tub+myo-fem line was crossed to a Cas9-expressing line, resulting in RNA-guided mosaic target gene mutations throughout mosquito development.
Dr. Akbari highlights the beauty of CRISPR technology for this work, describing how it allowed them to carry out what the group first coined as lethal biallelic mosaicism or sterile mosaicism, which essentially creates a mosaic animal with targeted mutations in enough of these alleles to ensure complete penetrance of the desired phenotype.
»Mechanistically, this technology demonstrates a fundamental advance in genetics by which somatic biallelic disruptions in essential genes, that previously conferred recessive phenotypes, get simultaneously converted by pgSIT in many somatic cells resulting in dominant fully penetrant phenotypes in a single generation«, said Dr. Akbari.
Lessons from cage trials: it is all a numbers game
The real-world application of a conventional SIT system is contingent on continuous releases of sterile males into the wild. A dedicated facility is needed to handle and generate the genetically modified sterile mosquitoes in a controlled environment.
To test their system, the team carried out multigenerational cage trials demonstrating the ability of released pgSIT adults or eggs to suppress mosquito populations.
Releasing adults at high pgSIT:WT ratios (20:1, 40:1) eliminated the tested populations by generation 3, while egg releases at the same thresholds accomplished elimination by generation 6. pgSIT:WT is the ratio of released genetically-modified mosquitoes/eggs vs. WT mosquitoes/eggs. This ratio simulates the releasing of X number of pgSIT mosquitoes for each wild mosquito in the environment, in this case 20 or 40 pgSIT mosquitoes for each wild mosquito (see Figure 2).
Suppressing populations in a safe and controllable fashion
As a post-doc at Caltech with Bruce Hay, Dr. Akbari was involved in developing the first CRISPR homing-based gene drives back in 2013. Having witnessed first-hand the lack of governance around gene drives and fears about the inherent risks and safety, addressing the main concerns with the use of gene drives is a top priority for him today:
»This technology that we developed here (…) gives us complete control, enables us to safely suppress and potentially eliminate disease transmitting populations. This is in stark contrast with gene drives. A gene drive does not give you that control, you release the gene drive and evolution takes over«, he explains.
Dr. Akbari also points out that the evolutionary stability of the new system is another major selling point: »pgSIT requires two breeding strains: one expressing Cas9 and the other expressing gRNAs. The system is therefore only active in the generated sterile progeny, so there really isn’t really a chance for resistance to evolve. Unlike insecticides or gene drives, which persist in the population and may provoke resistance, the released sterile males are unable to reproduce and therefore mutations in the target sequences which naturally exist in the wild will not affect the pgSIT system. Moreover, the CRISPR-edited sequences are gone after one generation because all the individuals released do not contribute to the gene pool and are essentially dead ends.«
The programmable nature of CRISPR also ensures a species-specific approach, leaving non-target organisms unaffected while simultaneously achieving precise targeting of genes without affecting fitness, all of which bolsters future field applications of pgSIT.
Scalability is the secret to global reach
The inability to transport fragile sterile adult mosquitoes limits the scalability of many population control technologies. Releasing eggs rather than adults into the environment may be a game changing solution, but such an approach cannot be done with conventional SIT, since half of the eggs will be females.
pgSIT solves this problem with vast potential for scalability. By mass rearing the two strains in a facility and crossing them together, the females will produce around 450 eggs each. Owing to the flightless phenotype of the pgSIT females (see Video below), the females will be unable to survive/transmit pathogens in the wild, while the sterile males will be able to find females and mate, resulting in suppression of the population.
Taking advantage of the eggs’ properties – they can be dried out and remain viable for one year – facilitates storage and transportation across the world. »The eggs simply need to be either released into a body of water to hatch directly into the environment, or hatched in rearing containers which would require the mosquitoes to fly out of the container to reach the environment – completely preventing the release of flightless females. This gives us a power we never had before. We can create fit organisms using CRISPR. We can store eggs and release eggs, which enables scalability«, elaborates Dr. Akbari.
Video description: Timelapse of myo-fem mutant flight. Cages consisting of myo-fem mutant ♀’s, myo-fem mutant ♂’s, WT ♀’s, and WT ♂’s were recorded over 5.5 minutes. The cages were occasionally tapped to stimulate movement. The myo-fem phenotype is visible from 2.20 minutes onwards. Symbols:♀= female, ♂= male.
A long, but exciting road ahead
Although the introduction of CRISPR-edited organisms into the environment will no doubt face regulatory challenges, Dr. Akbari is convinced it can be done: »SIT has been widely accepted. With pgSIT, you are simply releasing a sterile male. Yes, it is going to be genetically modified, but there are other examples of genetically-modified sterile male usage«.
The team is already working on the next steps in the project, with plans to test pgSIT on islands with sizeable mosquito populations and predominance of natural breeding sites. They will try to demonstrate that the system is scalable in a factory ensuring high quality standards during pgSIT strain production. They will also carry out surveillance in the wild, and use mathematical modelling in collaboration with the Marshall Lab at UCB to determine whether or not the level of scalability is feasible to effectively control the population. Dr. Akbari is optimistic that positive results will help him to engage with regulators and the public to seek approval for a field trial.
The shockwaves of this real-world solution extend beyond the use of CRISPR technology to control local mosquito populations. This work is an example of how CRISPR may eventually be used to address global disease burdens. By mass producing, distributing, and introducing pgSIT eggs across the world, it will be possible to introduce modified vectors into the wild at a global scale. As Dr. Akbari puts it: »This is potentially one of the biggest applications of CRISPR to date once we actually get it into the field«.
Link to the original article in Nature Communications:
Suppressing mosquito populations with precision guided sterile males.
Pedro Pais is a molecular biologist and microbiologist based in Portugal.
Tags
ArticleInterviewNewsInfectious diseaseVector-Borne Diseaseprecision guided Sterile Insect Technique (pgSIT)Sterile Insect Technique (SIT)
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.








