Double Knockout of Two Inflammatory Regulators Leads to Highly Effective T-Cell Therapy in Solid Tumours

Chimeric antigen receptor (CAR-T) cell therapies have contributed greatly to improved outcomes for individuals with haematological malignancies, but their effectiveness in combating solid tumours has lagged behind. One major reason for this is T cell exhaustion in the immunosuppressive tumour microenvironment, which limits the proliferation and function of the therapeutic cells in vivo.
In their latest study, members of Carl June’s lab at the University of Pennsylvania’s Center for Cellular Immunotherapies have demonstrated that double knockout of two key genes can enhance the ability of T cells to destroy cancer cells.
The study, published in PNAS, examines the effect of knocking out the Regnase-1- and Roquin-1-encoding genes in T cells, which significantly improves the long-term proliferation and tumour-clearing capacity of both CAR-T and TCR-T cell therapies in vivo in xenograft mice. David Mai, now a fourth-year PhD candidate, saw the project through from start to finish under the expert guidance of Dr. Neil Sheppard, who leads June’s T cell engineering lab.
As Mai comments, the complete clearance of solid tumours in mice by the double-knockout CAR-T cells was an incredible result:
»It was surprising how potent [the therapy] worked in the xenograft model, especially because we were using relatively low doses. In the case of the CAR-T cell therapy, we were definitely surprised that double knockout of these two genes had such a stark effect.«
T cell exhaustion and the problems in targeting solid tumours
T cell exhaustion is one of the key factors limiting the successful application of adoptive cell therapies such as CAR-T and TCR-T cell therapies in treating solid tumours, owing largely to the immunosuppressive tumour microenvironment.
»CAR-T cell therapies have been really successful in treating haematological malignancies, specifically B cell malignancies – for these we have really good targets that have led to approved products. Generally, in haematological malignancies, T cells are easily able to locate the cancer cells, and they're not dealing with a high degree of immune suppression. Unfortunately, when you move into solid tumours, that tends not to be the case,« Sheppard explains.
There have been many varied efforts to combat the problem of T cell exhaustion and increase their potential in treating solid tumours, from studies of the epigenetic mechanisms contributing to T cell exhaustion, to inhibition of immune checkpoints.
»Our focus was to try to address some of the challenges that T cell therapies faced in solid tumours. The idea was, if we take off the brakes in these T cells, we can make them more potent and more pro-inflammatory, so they will be able to mount a more effective anti-cancer response,« Mai elaborates.
Regnase-1 and Roquin-1 enhance T cell inflammatory function
In their study, Sheppard, Mai, and the team targeted two intrinsic inflammatory regulators, Regnase-1 and Roquin-1. Perhaps unsurprisingly, it was in vivo CRISPR knockout screens that allowed these genes to be identified as targets.
These two proteins play key roles in modulating inflammatory gene expression in T cells by degrading specific transcripts of inflammatory genes. Disruption of Regnase-1 and Roquin-1 leads to hyperinflammation – which is exactly what T cells need to persist in solid tumour environments. The first step for David Mai and his colleagues was to perform knockouts of Regnase-1 (Reg1-KO), Roquin-1 (Roq1-KO), or both (DKO) to examine the effects on the inflammatory profile of T cells.
They chose two clinical-stage T cell therapies directed against solid tumours with which to test their method: mesothelin-specific ‘mesoCAR-T’ cells and NYESO-specific ‘NYESO TCR-T’ cells. Mesothelin is a tumour-associated protein that is often overexpressed in pancreatic and ovarian cancers, while NYESO is a cancer germline antigen that is expressed in certain sarcomas and various other cancers.
»The main reason for our choice of models was that they had been used in our lab before, so it was easy to set up and use those models. And since we were trying to figure out whether this could potentially be a viable translational strategy, we also wanted to pick models that were actively being clinically pursued,« Mai explains.
DKO T cells can achieve complete tumour clearance in vivo
Interestingly, it was some of the earliest assays in the study that proved to be a key challenge. The team were trying to examine the difference between the knockout (KO) cells and normal cells, when the cells had already been activated. Sheppard explains:
»When T cells are activated, they naturally degrade the Regnase-1 and Roquin-1 proteins. That's part of the how these proteins work - they keep cells in a low inflammatory state when they're not activated. After activation, Regnase-1 and Roquin-1 get depleted, which means there isn't much of a phenotypic, functional difference between a ‘natural’ T cell and a Regnase-1 and Roquin-1 knockout T cell in a short term assay.«
After seeing only marginal differences in the KO cells compared to controls, and even wondering if they should continue with the research, the team decided to also examine the effects of KOs on resting T cells. Fortunately, that was where they saw big differences in the baseline activation profile of the edited cells compared to the control (mock) cells, and they proceeded with the study.
The team next evaluated the anti-tumour activity of the edited NYESO TCR-T cells and mesoCAR-T cells in vivo in xenograft mice. For the NYESO TCR-T cells, Reg1-KO and DKO cells displayed the greatest anti-tumour activity, with DKO cells showing greatest expansion in peripheral blood, while Roq1-KO performed similarly to mock cells.
In the edited mesoCAR-T cells, Reg1-KO and Roq1-KO cells both displayed enhanced anti-tumour function and were able to clear tumours in 85% and 59% of mice, respectively. The DKO mesoCAR-T cells performed even better – they achieved complete tumour clearance in an incredible 92% of mice (See Figure 1 below). The DKO cells were also able to penetrate and accumulate the most in the tumours.
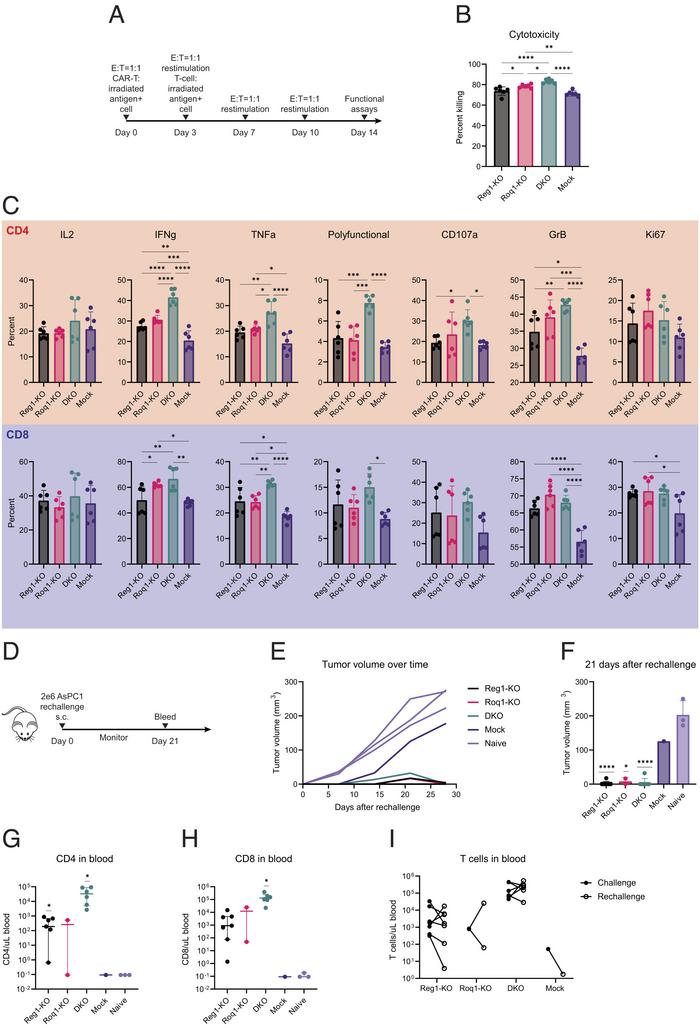
Figure 1: Regnase-1 and Roquin-1 double knockout enhances functional persistence. (A) In vitro restimulation assay to assess functional persistence. (B) Cytotoxicity of serially restimulated CAR-T cells at various E:T ratios. (C) Cytokine expression of serially restimulated CD4 (Top) and CD8 (Bottom) T cells. (D) In vivo mouse experiments to evaluate persistence of knockout CAR-T cells and response to tumour rechallenge. (E,F) Reponses to tumour rechallenge by knockout and mock CAR-T cells following at least a month from primary tumour clearance. (E) Tumour growth over time after rechallenging previously treated mice. (F) Tumour sizes 21 d after rechallenge. (G,H) Quantification of CD4 (Left) and CD8 (Right) T cells in peripheral blood 21 d following tumour rechallenge. (I) Changes in peripheral T cell numbers between 21 d after initial tumour challenge and 21 d after tumour rechallenge in rechallenged tumour-cleared mice. Mai et al. PNAS (2023) doi.org/10.1073/pnas.2218632120
Overcoming toxicity and exploring clinical translation
There was one downside to having such effective Reg1-KO and DKO mesoCAR-T cells with no brakes on their inflammatory function: toxicity. Several of the mice that had been treated with the cells died after their tumour burden had been cleared, but as Sheppard explained, this was not due to graft versus host disease (GvHD) as they first expected.
A control experiment in which they knocked out the endogenous TCR of the edited cells - which is essential for mediating GvHD – still resulted in toxicity. Further investigations revealed that the toxicity was due to over-proliferation of the T cells in the blood of the xenograft mice, who lacked T cells of their own. To combat toxicity, the team titrated down the dose of the edited T cells.
»We wanted to see whether if we lowered the starting dose, we could mitigate this toxicity, but also still retain similar anti-tumour function. And it turns out that even at lower doses, the edited cells were still able to clear tumours better than doses that were five or 10 times higher in unedited T cells,« Mai says.
The toxicity may not occur in human patients who have existing T cell populations, however, the team will likely need to find a more solid solution before any human testing can begin.
»We're looking at more control. One solution is the inclusion of a pharmacologically activated suicide switch, so that you don't have to leave these therapeutic cells in the body forever. Or if they do start to cause trouble, you can wipe them out,« Sheppard adds.
The results of the study certainly demonstrate the potential of these DKO T cells for the treatment of solid tumours, and the team continues to pursue this avenue by gathering pre-clinical data. However, as Sheppard concludes, the results also highlight the strength of CRISPR as a tool for identifying targets and allowing these complex edits to be viable:
»It's really exciting to live and work in a time where these tools are available. CRISPR is still a relatively new tool and just look at all of the potential that it's unlocking – there are probably good reasons why these genes weren't discovered until there was a really nice clean tool like CRISPR.«
Link to the original article in PNAS:
Rebecca Roberts is a molecular biologist and science writer/communicator based in Queensland, Australia.
To get more CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
CLINICAL TRIALS
Sponsors:
Wave Life Sciences Ltd.







